盐度是影响贝类生长发育的重要因子。近年来,气候变化和人为活动等因素导致潮间带盐度异常,低盐胁迫事件频发。低盐环境会导致贝类渗透调节失衡[1],机体通过离子转运蛋白(如Na+/K+-ATP酶)的活性调整来维持细胞内外渗透压平衡,且这一过程会消耗大量能量,进而抑制生长速率与摄食效率[2],使得贝类生长受到抑制甚至死亡[3]。同时,低盐胁迫引发细胞脱水或过度吸水,导致离子通道活性异常,细胞膜稳定性降低,生理功能受损[4]。低盐环境还会抑制贝类的免疫反应,导致病原体易感性增加,贝类死亡率升高[5]。因此,筛选耐低盐的优良品系,对优化养殖品种、拓展低盐地区养殖空间具有重要意义。
家系选育可根据整个家系的性状优异情况对抗逆性状进行选育。张兴志等[6]采用家系选育的方法挑选出了5个可以用于后续耐高盐性状选育的香港牡蛎家系;Mccarty等[7]通过培育家系对美洲牡蛎(Crassostrea virginica)的耐低盐性状进行了评估;段毓佳等[8]通过家系评估和选育后,最后得到了具有优良耐低氧性状的凡纳滨对虾(Litopenaeus vannamei)家系材料。由此可见,通过家系选育可以提升物种的耐低盐性能。
缢蛏(Sinonovacula constricta)作为中国重要的双壳类经济物种,属于四大海水养殖贝类之一。目前,针对缢蛏抗逆性的研究多集中于高温、高盐和氨胁迫环境下的行为响应及生理机制[9-12]。关于缢蛏不同家系在低盐胁迫下的表型差异及遗传潜力尚未系统阐明。本研究中以缢蛏不同家系为研究对象,通过对比其在低盐胁迫下的组织损伤、细胞凋亡、抗氧化酶活性及Na+/K+-ATPase活性等指标,评估了家系间耐低盐性能的差异,以期为耐低盐新品系选育提供理论依据,同时为低盐水域养殖实践提供技术参考。
1 材料与方法
1.1 材料
2023年9月开始相关试验,选取体质量为(10.16±1.15)g,壳长为(5.39±1.12)cm的福建宁德缢蛏群体作为亲本。
1.2 方法
1.2.1 建立家系 缢蛏家系构建在浙江省三门县育苗基地开展,参考Chen等[11]方法,首先将挑选符合标准的健康缢蛏作为亲贝,置于阴凉处风干6 h,然后转移到繁殖专用产床上,悬挂在繁殖池(盐度为12.0±0.8,温度为22.0 ℃±0.2 ℃)中,并通过池底持续的气流来刺激缢蛏排放精子和卵子;将正在排精产卵的缢蛏个体分别转移到装有过滤海水的烧杯中;待精子和卵子完全排放后,将精子均分为3份,分别与来自不同母本卵子混合,以此完成人工授精过程。总共建立了30个家系。
1.2.2 家系培育与池塘养殖 每个家系单独养殖于200 L塑料箱中,开口饵料为等鞭金藻(Isochrysis galbana),投喂至壳顶幼虫阶段后投喂等鞭金藻和牟氏角毛藻(Chaetoceros muelleri)混合藻液,每日换水。室内培育60 d后,于11月将所有家系转入池塘养殖。每个家系养殖面积为1 m×2 m的矩形区域,四周拉网隔开各家系并防止敌害入侵。养殖密度为300~400 粒/m2,盐度为19~22。
1.2.3 低盐胁迫试验 室外养殖5个月后,将缢蛏家系采捕。本试验选取F8、F13、F14、F23、F24、F28 6个家系,每个家系设置100粒缢蛏,胁迫盐度为2,对照盐度为20。试验低盐水体用砂滤海水和淡水配置,期间每天全部换水,并投喂小球藻和牟氏角毛藻混合藻液。试验周期内每小时观察一次缢蛏存活状态,移除死亡个体并记录死亡时间点(精确至分)。体质量采用电子天平(精度0.01 g)测定,壳形态参数(SL、SH、SW)采用数显卡尺(精度0.01 mm)测量,操作前用滤纸吸除缢蛏体表水分。死亡率试验终止时间点为任一家系存活率为0。胁迫试验结束后再挑选每个家系90粒缢蛏(平均壳长为4.6 cm±1.2 cm,体质量为7.0 g±1.9 g)。胁迫盐度为2,对照组盐度为20,每组设置3个重复。试验水体用砂滤海水和淡水配置,每日换水。胁迫开始后分别在 0、6、12、24、48、72 h对每个家系缢蛏的鳃组织取样,用于抗氧化酶活性及Na+/K+-ATP酶活性测定,样本首先放入液氮中,再转至-80 ℃冻存;在 0、24、48、72 、96 h取出用于细胞凋亡和组织损伤观察的鳃组织样本,组织放入装有1 mL多聚甲醛的离心管中,4 ℃下保存。
1.2.4 死亡和存活能力分析 试验期间内系统记录各家系死亡个体数及相对存活率(relative survival rate,RSR),其中,RSR定义为试验终止时存活个体数与初始投放量的百分比值。采用皮尔逊(P)和斯皮尔曼(S)相关系数用于分析死亡时间与壳形态特征及体质量的关联性。
构建Kaplan-Meier生存函数曲线生存函数进行生存差异分析。生存概率函数计算公式为
(1)
式中:ti为第i天发生死亡事件;di为对应时间点死亡个体数;ni为t i前存活个体数。
1.2.5 石蜡切片的制备及HE染色 由多聚甲醛固定好后,鳃组织经固定、脱水,用二甲苯透明浸蜡及石蜡包埋,切片厚度6 μm,用作HE染色,中性树胶封片。
1.2.6 Hoechst凋亡染色及观察 包埋后的组织经切片、脱蜡复水处理后,按照索莱宝Hoechst染色试剂盒进行染色(北京索莱宝科技有限公司),在体式荧光显微镜下观察细胞凋亡情况。
1.2.7 抗氧化酶活性及Na+/K+-ATPase活性测定 组织质量和提取液体积按比例进行冰浴后匀浆,4 ℃下以8 000 r/min离心10 min,取上清,按索莱宝超氧化物歧化酶(SOD)活性、过氧化氢酶(CAT)活性、Na+/K+-ATPase(NKA)活性和丙二醛(MDA)含量检测试剂盒说明书操作(北京索莱宝科技有限公司)。
1.3 数据处理
试验数据用平均值±标准差(mean±S.D.)表示,采用SPSS 22.0统计软件进行独立样本t检验比较检验数据差异的显著性,显著性差异设为0.05。
2 结果与分析
2.1 低盐条件下缢蛏家系存活时间和表型性状相关性
低盐胁迫试验共持续17 d,F8家系在最后一天全部死亡。图1为低盐胁迫后缢蛏的Kaplan-Meier存活曲线。死亡率较高的3个家系其存活曲线与所有死亡个体的存活曲线相似。在第11天后,死亡间隔相对较短,缢蛏的存活能力显著降低。死亡率较低的3个家系的存活曲线较为平缓,且开始出现死亡个体的时间也有所差异,表明较为耐受的3个家系的耐低盐能力仍有所不同。
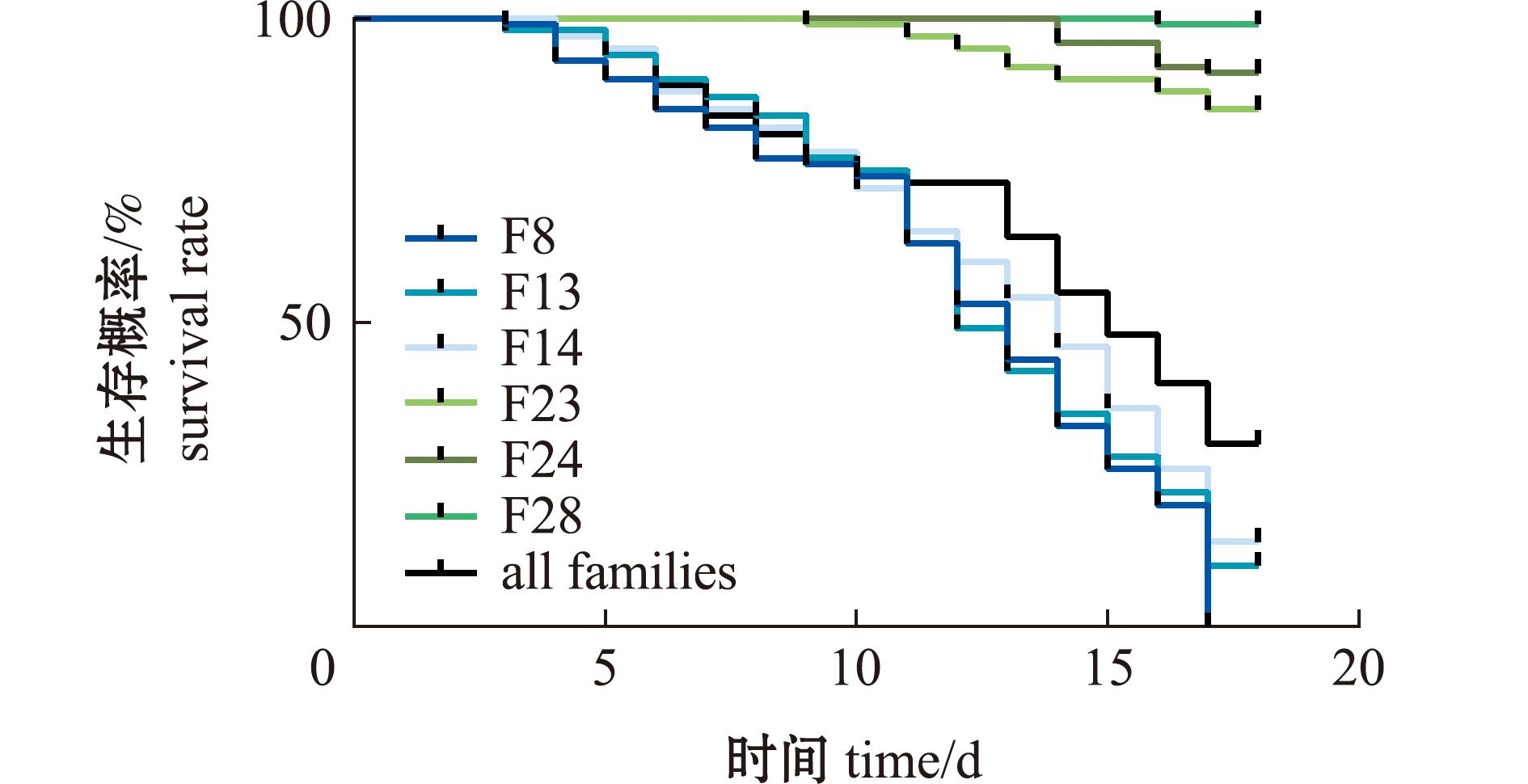
图1 低盐胁迫下缢蛏死亡和Kaplan-Meier生存曲线
Fig.1 Kaplan-Meier survival curves of Sinonovacula constricta under low salinity stress
在低盐条件下,6个家系的缢蛏存活时间和生长性状的皮尔逊(P)和斯皮尔曼(S)相关性见表1。从表1可见,各家系缢蛏存活时间与SL、SH、SW、BW的皮尔逊和斯皮尔曼相关性除F23外差异不大。其中,F8缢蛏存活时间与生长性状整体呈现负相关,F23、F24、F28呈现正相关。将所有死亡缢蛏个体做一个整体统计,缢蛏存活时间与生长性状的皮尔逊和斯皮尔曼相关性均是正相关关系。其中,缢蛏存活率和SW的皮尔逊相关性极显著 (P<0.01),而SW和BW的斯皮尔曼相关性显著 (P<0.05)。
表1 低盐条件下缢蛏存活时间与表型之间的相关系数
Tab.1 Correlation coefficients between survival time and phenotypic traits of the Sinonovacula constricta under low-salinity stress
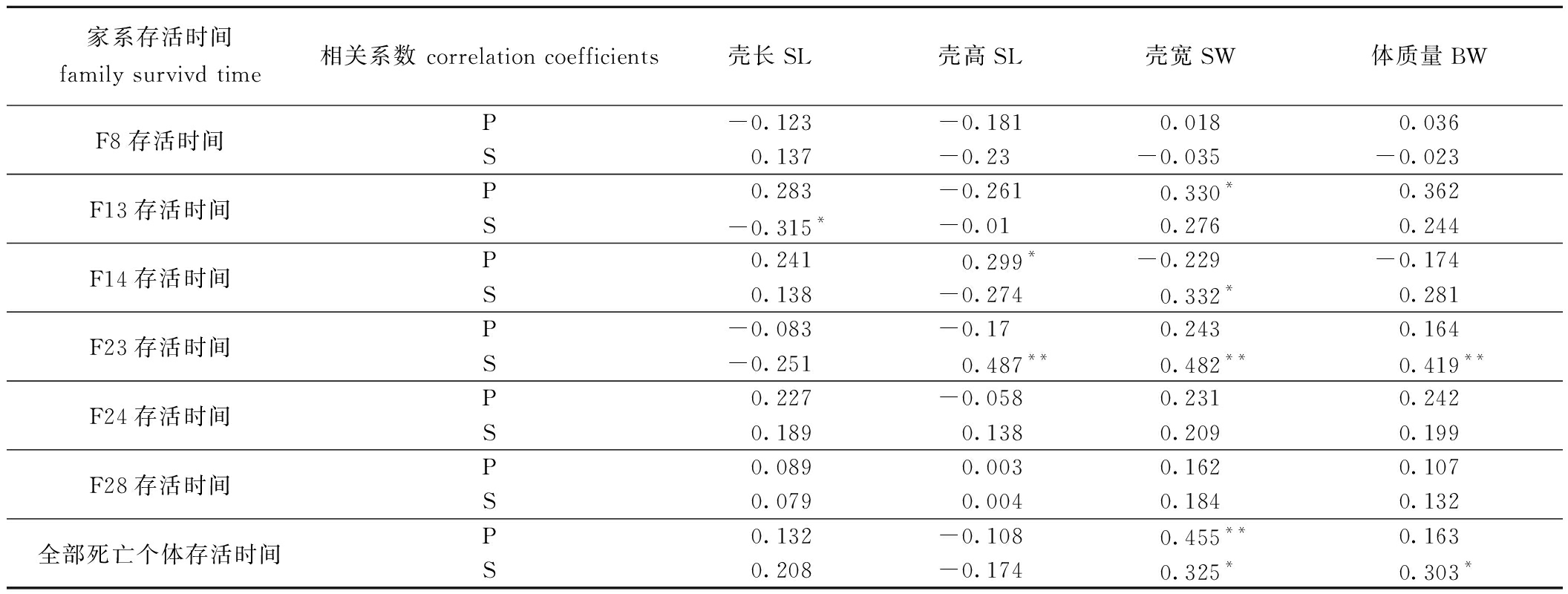
家系存活时间family survivd time相关系数 correlation coefficients壳长SL壳高SL壳宽SW体质量BWF8存活时间P-0.123-0.1810.0180.036S0.137-0.23-0.035-0.023F13存活时间P0.283-0.2610.330*0.362S-0.315*-0.010.2760.244F14存活时间P0.2410.299*-0.229-0.174S0.138-0.2740.332*0.281F23存活时间P-0.083-0.170.2430.164S-0.2510.487**0.482**0.419**F24存活时间P0.227-0.0580.2310.242S0.1890.1380.2090.199F28存活时间P0.0890.0030.1620.107S0.0790.0040.1840.132全部死亡个体存活时间P0.132-0.1080.455**0.163S0.208-0.1740.325*0.303*
注:*表示显著相关(P<0.05),**表示极显著相关(P<0.01)。
Note:* means significant correlations(P<0.05),**means very significant correlations(P<0.01).
2.2 低盐条件下缢蛏鳃组织损伤情况
通过苏木精-伊红(HE)染色观察发现,低盐胁迫对缢蛏鳃组织结构的影响在各个家系间存在差异(图2)。F13、F14家系呈现渐进性损伤特征:72 h时部分鳃丝出现肿胀,鳃腔扩张,胁迫至96 h时,鳃丝出现破损,相邻鳃丝间距缩小。F8家系在48 h前组织结构未见明显改变,72 h时鳃丝肿胀,96 h时鳃体积变大。F23家系在72 h时局部鳃丝出现轻微水肿,但未影响气体交换功能,至96 h时损伤程度仍低于上述3个家系。F24、F28家系在整个96 h胁迫周期内保持鳃丝结构完整,鳃丝排列密度维持正常水平,未出现明显结构改变。
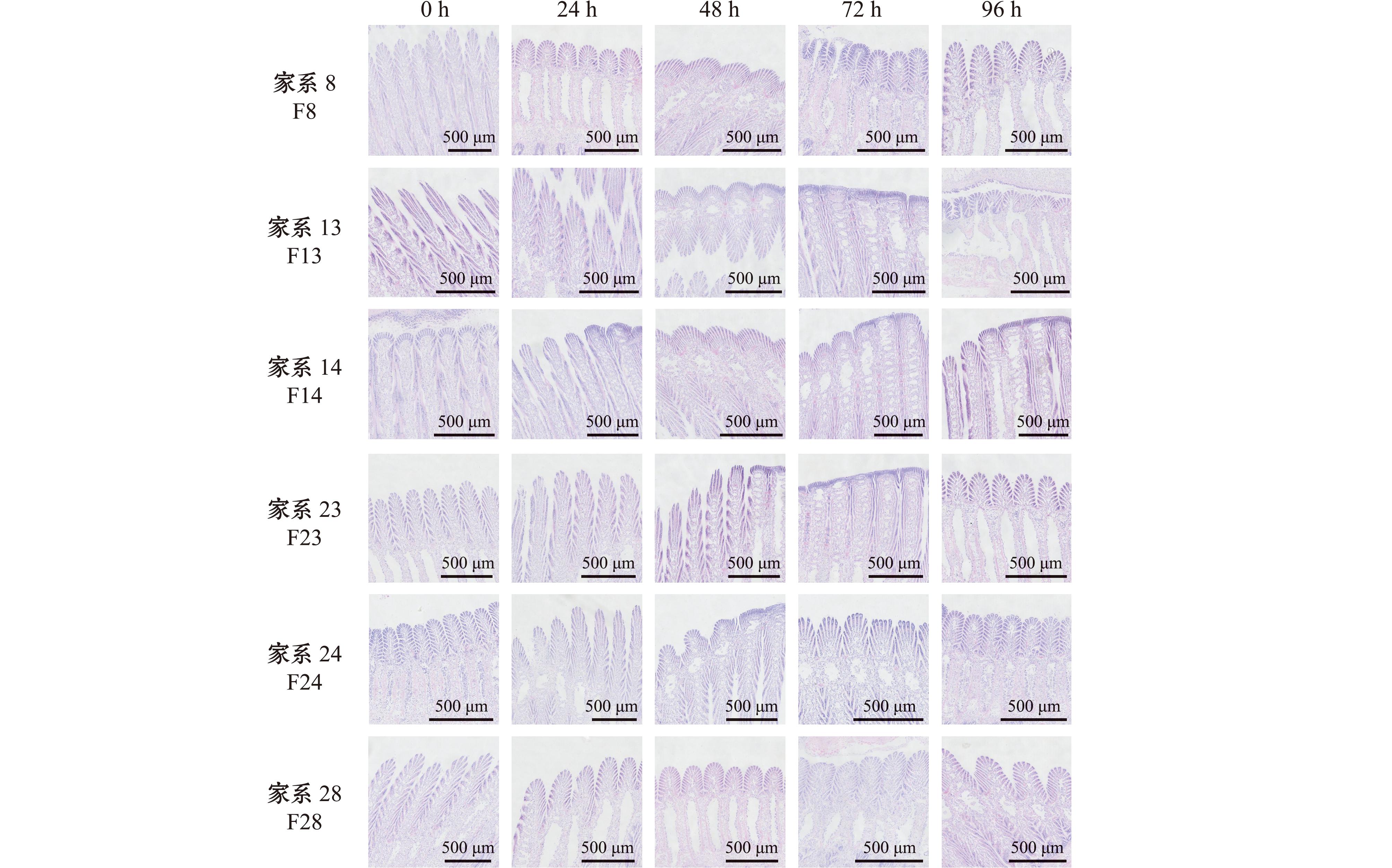
图2 低盐条件下不同家系缢蛏的鳃组织结构
Fig.2 Gill tissue structure in different families of Sinonovacula constricta under low salt salinity stress
2.3 低盐条件下缢蛏鳃细胞凋亡情况
通过Hoechst 33342染色观察低盐胁迫下各家系缢蛏鳃组织细胞凋亡情况。从图3可见,F8、F13、F14家系细胞核呈现致密亮蓝色斑块,面积较正常核有所缩减;视野可检出凋亡小体,呈致密浓染状,颜色发白,聚集于鳃丝损伤区域;鳃丝基部向顶端呈递增趋势,顶端区域凋亡较多。F23家系凋亡细胞比例较上述3个家系略少,凋亡小体主要分布于鳃腔扩张区域。F24、F28家系中核染色质均匀分布,呈弥散蓝色荧光,凋亡细胞比例较少;偶见零星凋亡小体,主要分布于鳃丝边缘。
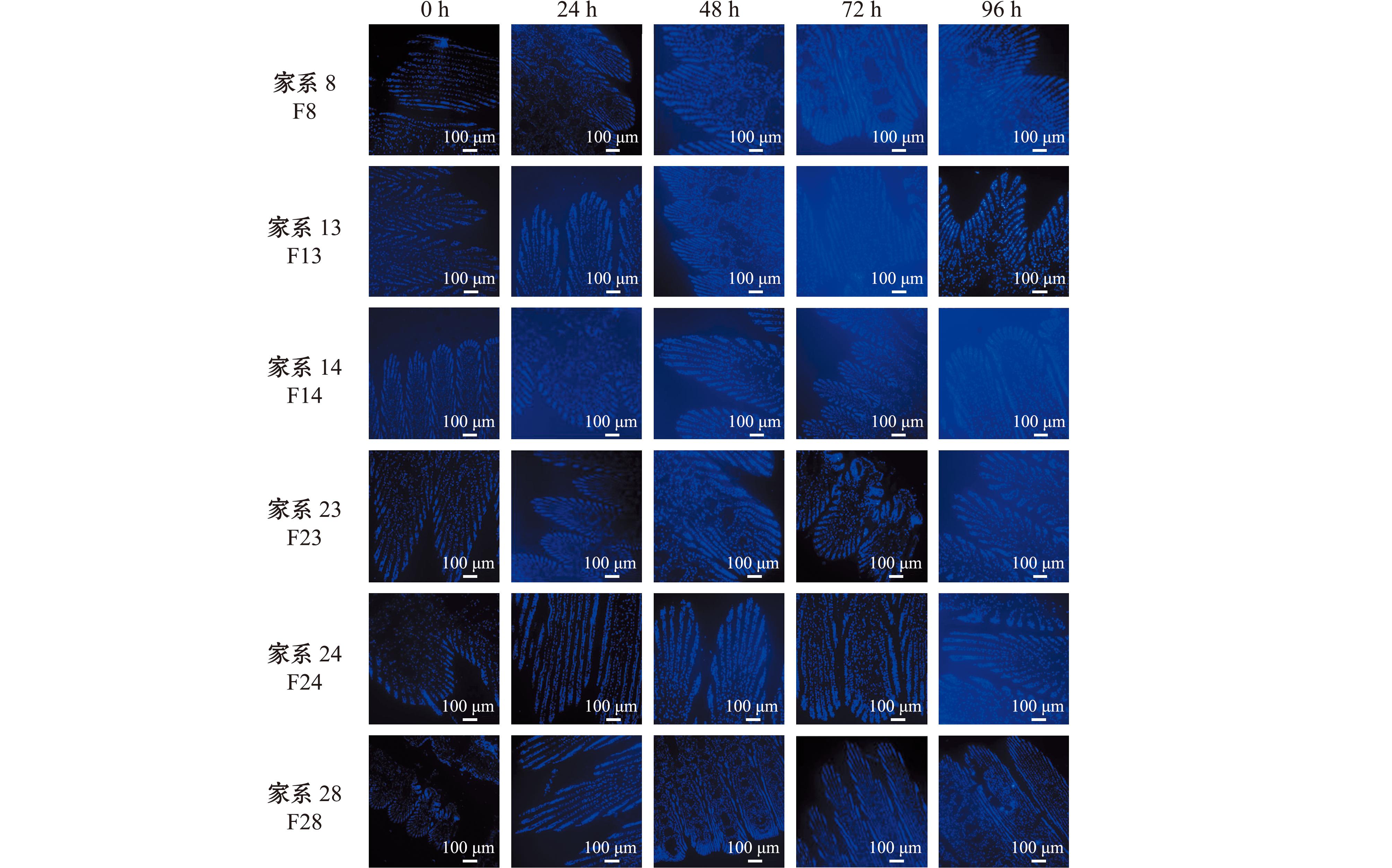
图3 低盐条件下不同家系缢蛏的鳃组织细胞凋亡情况
Fig.3 Apoptosis of gill tissue cells in different families of Sinonovacula constricta under low salinity stress
2.4 鳃组织钠钾ATP酶(NKA)活性
从图4可见,所有家系NKA活性在低盐胁迫0~12 h内均呈持续下降趋势。至24 h时,F23、F24、F28家系表现出典型的应激响应模式——酶活性先呈显著升高的趋势,随后进入渐进衰减阶段;而F8和F13家系则持续维持下降趋势(衰减速率分别为每小时0.8、0.5 U/g quality),且F8的NKA酶活性水平始终低于F13。尤其,24 h后F23、F24、F28与其余3个家系间的酶活性差异随时间延长逐步收敛,72 h时组间差异较24 h有所降低,提示长期胁迫下不同家系渗透调节策略存在趋同进化现象。
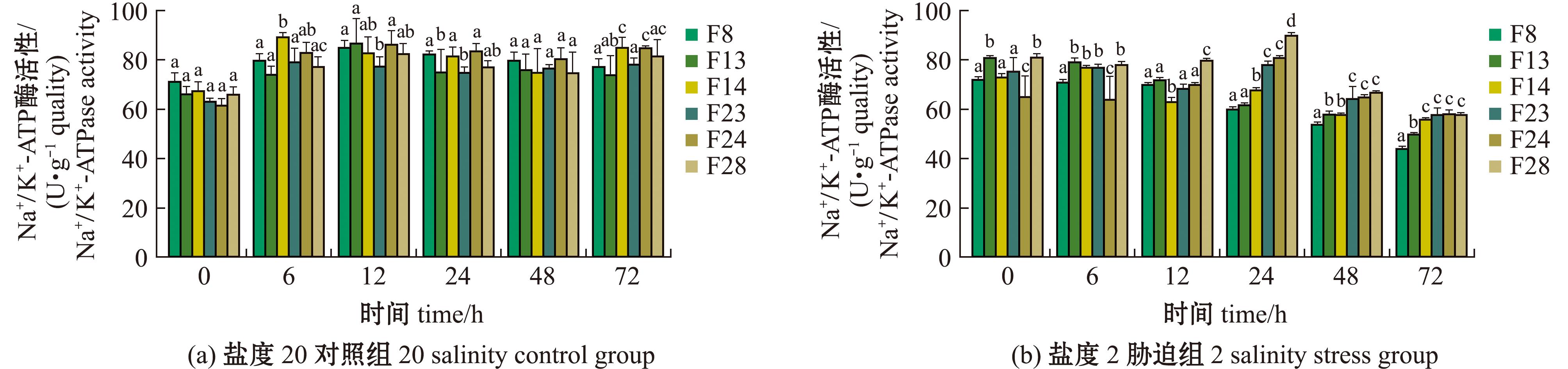
图标有不同小写字母者表示组间有显著性差异(P<0.05),标有相同小写字母者表示组间无显著性差异(P>0.05),下同。
The means with different letters within the picture are significant differences at the 0.05 probability level,and the means with the same letters are not significant differences,et sequentia.
图4 缢蛏各家系Na+/K+-ATP 酶活性水平
Fig.4 NKA enzyme activity levels in different families of Sinonovacula constricta
2.5 鳃组织抗氧化酶活性
2.5.1 超氧化物歧化酶(SOD)活性 从图5可见,在低盐组和对照组中,所有家系整体SOD活性均呈现双相响应模式——初始阶段(0~24 h)快速升高,随后(24~72 h)逐步衰减。低盐组酶活性峰值显著高于对照组,其中低盐条件下F24在24 h时达到最高值(196.6±12.5)U/g quality,较初始水平提升401.5%。胁迫后期(48~72 h),F8、F13、F14家系表现出较低的衰减速率(每小时0.8~1.8 U/g quality),但其酶活性水平始终低于F23、F24、F28家系。
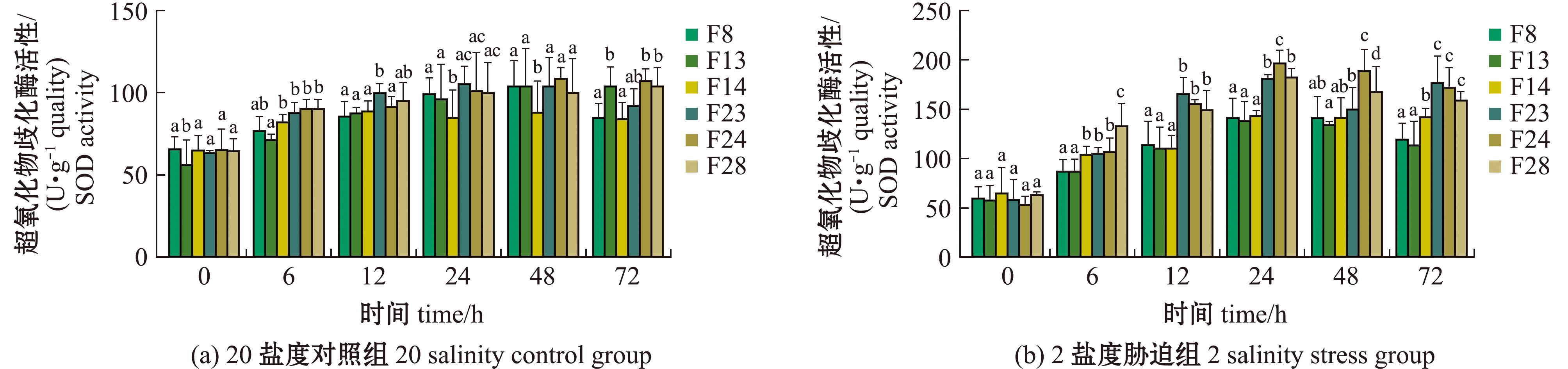
图5 缢蛏各家系SOD酶活性水平
Fig.5 SOD enzyme activity levels of different families of Sinonovacula constricta
2.5.2 丙二醛(MDA)含量 从图6可见,在低盐胁迫周期内,所有家系MDA含量呈现典型的“V”型动态特征——初始阶段(0~12 h)显著下降(降幅达18.6%~52.3%,P<0.05),在12~24 h内达到最低点F8:(7.49±0.45)mmol/g quality;F28:(12.71±0.61)mmol/g quality,随后MDA含量提升。至试验终点,F8的MDA含量升至峰值(31.93±1.02)mmol/g quality,显著高于F23(13.36±0.78)mmol/g quality(P<0.001),且F8、F13、F14的MDA含量整体水平较其他3个家系更高。
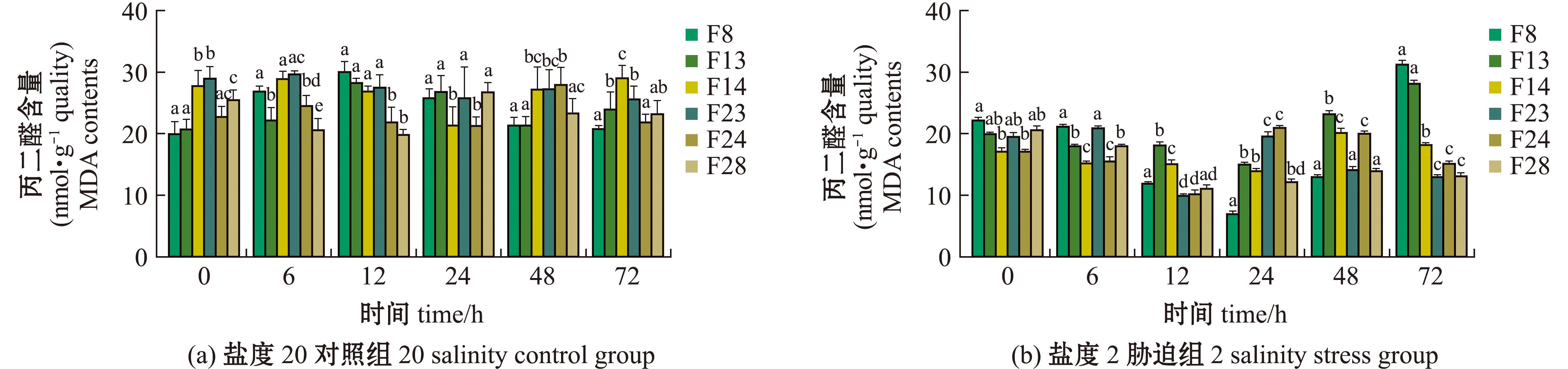
图6 缢蛏各家系MDA含量
Fig.6 MDA contents of different families of Sinonovacula constricta
2.5.3 过氧化氢酶(CAT)活性 从图7可见,盐度2胁迫条件下,各家系的CAT活性呈现时相性差异(P<0.05),F8、F13、F14在0~24 h内CAT活性持续下降,其中,在12 h时达最低值(F8:367.21±27.51)U/g quality;F13:(378.30±38.26) U/g quality,较初始水平降低52.3%~55.7%(F=18.43,P<0.001);24~48 h内出现短暂回升,但72 h时再次下降至胁迫中后期水平。胁迫试验期间F23、F24、F28活性先降低后趋于稳定,3个家系均在12 h达最小值,较初始值降低20%以上,并且在48~72 h维持高活性水平(均值>700 U/g quality),在72 h时仍保持较高水平。
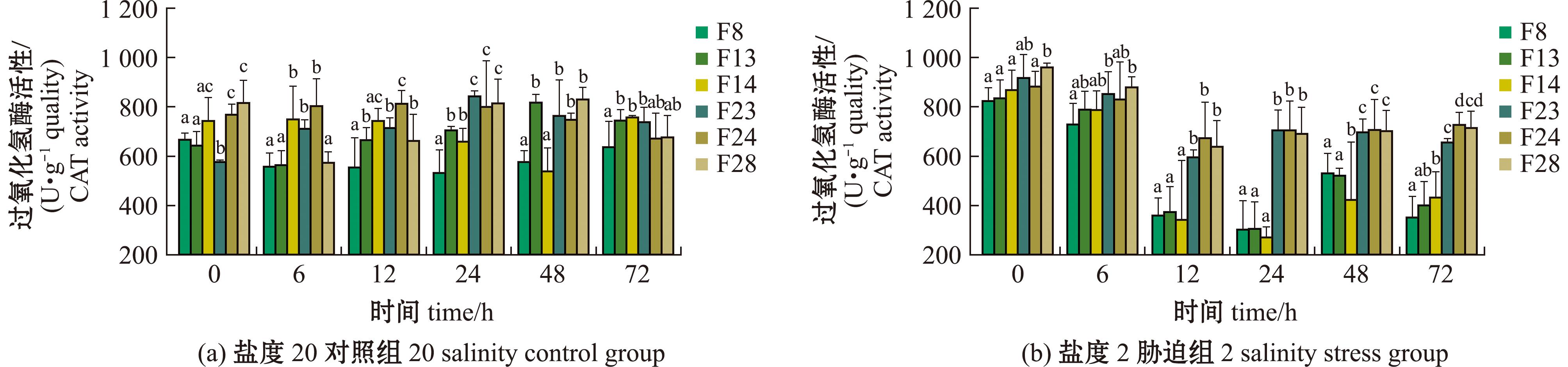
图7 缢蛏各家系CAT酶活性水平
Fig.7 CAT enzyme activity levels of different families of Sinonovacula constricta
3 讨论
3.1 低盐环境对缢蛏鳃组织结构的影响
缢蛏是一种滤食性贝类,能够大量过滤海水中的藻类 [13-14]。缢蛏鳃纤毛的规律摆动驱动水流,帮助滤食,若鳃结构受损,其滤食效率降低,导致能量摄入不足和生长停滞[15];同时,鳃组织是应对盐度波动的关键屏障,离子通道活性也依赖鳃完整性[16]。因此,鳃组织结构完整对缢蛏的生存、代谢及环境适应性具有重要作用。鳃丝结构的完整性可保障其滤食效率,本研究中F8、F13、F14家系因鳃丝肿胀和间距缩小,导致滤食效率降低,在长期低盐条件下生存时可能会因能量摄入少导致耐受性降低。因此在低盐度胁迫条件下,具有耐受性的贝类消耗较少的能量便可维持机体稳定,而更多的能量用于摄食,有助于个体快速生长[17]。
3.2 低盐条件下缢蛏鳃组织细胞凋亡的特征
组织结构的变化离不开细胞凋亡这一种程序性死亡现象[18]。该机制作为生物体的重要保护机制,可通过选择性清除受损细胞以应对环境压力[19]。研究表明,非生物逆境胁迫可显著增强细胞凋亡进程。本研究中发现,在急性低盐胁迫条件下,部分家系鳃组织呈现出渐进性细胞凋亡特征:初期凋亡信号由鳃基部向鳃丝区域扩散,中期鳃丝细胞群体出现广泛凋亡特征,至胁迫终期则检测到鳃丝细胞群体的完全凋亡现象,该时序性凋亡模式与已有报道具有一致性[20]。值得注意的是,有研究发现,拟穴青蟹(Scylla paramamosain)可通过调控活性氧(ROS)生成可有效抑制凋亡相关基因表达,从而显著降低细胞凋亡发生率,这为环境胁迫下的细胞保护机制研究提供了重要试验依据[21-22]。以上研究表明,组织修复能力与选择性凋亡调控共同维持了耐受家系个体的生理功能。
3.3 低盐环境对缢蛏鳃组织Na+/K+-ATP酶活性的影响
Na+/K+-ATP酶(NKA)是横跨质膜的一种酶类,通过消耗ATP来维持Na+、K+离子的交换,在机体离子调节中发挥着重要作用,对细胞的渗透调节、物质吸收(葡萄糖、氨基酸等)和跨细胞离子运动有重要影响[23]。该酶通过催化ATP末端高能磷酸键水解获得能量,驱动Na+与K+逆浓度梯度的主动跨膜转运,这种离子泵机制对维持细胞膜电位稳定性、调节细胞容积及保障物质转运等基础生理过程具有决定性作用[24]。作为细胞稳态的重要调节器,NKA通过精确调控胞内外离子浓度梯度,为神经冲动传导、肌肉收缩等基础生理过程提供必需的电化学梯度,其功能完整性是生物能量转换与细胞代谢平衡的关键分子基础。海洋无脊椎动物通过渗透调节机制维持体液稳态的核心环节,依赖于鳃上皮细胞基底膜系统中NKA的离子转运功能。研究发现,缢蛏在急性低盐胁迫初期NKA活性显著升高,机体通过增强离子泵功能以维持细胞内外的渗透压平衡的适应性反应[25],随着低盐胁迫时间的延长,NKA活性逐渐下降并趋于稳定,这可能与能量耗竭或细胞损伤有关,并最终导致渗透压调节能力减弱。彩虹明樱蛤的研究显示[26],低盐胁迫下NKA活性在48~72 h达到峰值后下降,这一模式可能也适用于缢蛏。这种盐度依赖性应答机制通过调控ATP水解驱动的Na+/K+跨膜转运效率,实现胞内离子稳态重建,为贝类应对盐度骤变提供了关键性的适应策略。本研究中发现,F23、F24、F28家系通过稳定维持鳃组织NKA活性(72 h时仍为初始值的85.3%±6.7%),有效调控胞内离子稳态,同时避免过度能量消耗。这种“精准调控”模式与F8、F13、F14家系形成鲜明对比:后者在胁迫初期(24 h)NKA活性代偿性升高至2.5倍,但可能伴随ATP含量骤降,最终引发p38 MAPK介导的线粒体凋亡途径激活[27]。这表明耐低盐性能的核心在于能量分配效率而非单纯渗透调节强度。
3.4 低盐环境对缢蛏鳃组织抗氧化酶活性的影响
低盐胁迫会诱导海洋贝类ROS过量积累,导致脂质过氧化和蛋白质损伤[26]。若胁迫强度超出阈值,抗氧化系统的代偿能力将崩溃,MDA含量急剧上升,引发细胞凋亡和组织功能障碍[28-29]。SOD和CAT活性在低盐胁迫后分别有所下降,导致MDA含量增加,MDA含量上升影响抗氧化酶对ROS的清除能力[30],引发鳃丝细胞膜脂质过氧化[31],导致空泡化面积增加[32]。抗氧化酶系统中CAT-SOD协同体系是生物体对抗活性氧损伤的初级防御屏障,当该核心酶系功能受损时,机体将代偿性激活其他抗氧化酶防御网络。研究表明,提升CAT和SOD的生物活性可显著降低脂质过氧化水平,从而延缓细胞膜结构完整性的破坏[33-35]。其中CAT通过催化过氧化氢(H2O2)的歧化反应,将其高效转化为水分子和氧气,这一生化过程对于清除毒性氧自由基具有关键性作用[36]。上述差异表明,抗氧化系统持续激活能力是影响家系耐盐性的关键,而短暂代偿性升高反而加剧能量危机[37-38]。
4 结论
1)本研究中通过对缢蛏家系死亡率、组织损伤、细胞凋亡、抗氧化酶活性及Na+/K+-ATPase活性测定,评价了6个家系的耐低盐水平。结果表明,不同缢蛏家系间耐低盐水平存在着较大差异。
2)试验中筛选3个具有低盐耐受性的家系,这些家系在各个水平都表现出了优秀的耐低盐性能,因此可考虑将这些家系作为后续选择育种的优势家系。
[1] 葛红星,李雯倩,柳佳玲,等.低盐对青蛤抗氧化酶和ATP酶的影响及碳酸酐酶基因的克隆与表达[J].中国水产科学,2021,28(8):968-977.GE H X,LI W Q,LIU J L,et al.Effects of salinity stress on antioxidase,ATPase,and carbonic anhydrase gene expression in Cyclina sinensis[J].Journal of Fishery Sciences of China,2021,28(8):968-977.(in Chinese)
[2] MASUI D C,MANTELATTO F L M,MCNAMARA J C,et al.Na+,K+-ATPase activity in gill microsomes from the blue crab,Callinectes danae,acclimated to low salinity:novel perspectives on ammonia excretion[J].Comparative Biochemistry and Physiology Part A:Molecular &Integrative Physiology,2009,153(2):141-148.
[3] 林瑞才,陈敏,林笔水.温度和盐度对海湾扇贝幼虫附着及变态的影响[J].台湾海峡,1989,8(1):60-67.LIN R C,CHEN M,LIN B S.Effects of temperature and salinity on attachment and metamorphosis of bay scallop larvae Argopecten irrdians(Lamarck)[J].Journal of Oceanography in Taiwan Strait,1989,8(1):60-67.(in Chinese)
[4] 牛东红,王宏蕾,李家乐.海洋贝类对盐度胁迫适应机制的研究进展[J].水产学报,2024,48(4):32-44.NIU D H,WANG H L,LI J L.Research progress on adaptation mechanism of marine shellfish to salinity stress[J].Journal of Fisheries of China,2024,48(4):32-44.(in Chinese)
[5] 杨东敏,张艳丽,丁鉴锋,等.高温、低盐对菲律宾蛤仔免疫能力的影响[J].大连海洋大学学报,2017,32(3):302-309.YANG D M,ZHANG Y L,DING J F,et al.Synergistic effects of high temperature and low salinity on immunity of Manila clam Ruditapes philippinarum[J].Journal of Dalian Ocean University,2017,32(3):302-309.(in Chinese)
[6] 张兴志,官俊良,何苹萍,等.香港牡蛎野生群体家系建立及耐高盐性筛选[J].南方农业学报,2019,50(2):385-390.ZHANG X Z,GUAN J L,HE P P,et al.Establishment of families and subsequent high salinity resistance testing for wild population of Crassostrea hongkongensis[J].Journal of Southern Agriculture,2019,50(2):385-390.(in Chinese)
[7] MCCARTY A J,MCFARLAND K,SMALL J,et al.Heritability of acute low salinity survival in the Eastern oyster (Crassostrea virginica)[J].Aquaculture,2020,529:735649.
[8] 段毓佳,谭建,栾生,等.凡纳滨对虾在低氧环境下存活性状的遗传参数评估[J].渔业科学进展,2024,45(1):138-147.DUAN Y J,TAN J,LUAN S,et al.Evaluation of genetic parameters for survival traits of Litopenaeus vannamei under hypoxic conditions[J].Progress in Fishery Sciences,2024,45(1):138-147.(in Chinese)
[9] 申奔龙,薛宝宝,孟德龙,等.缢蛏早期耐高温家系建立及抗氧化能力测定[J].浙江农业学报,2022,34(2):266-274.SHEN B L,XUE B B,MENG D L,et al.Establishment of a high temperature resistant family and determination of antioxidant capacity of razor clam Sinonovacula constricta[J].Acta Agriculturae Zhejiangensis,2022,34(2):266-274.(in Chinese)
[10] 李炼星,杜文俊,王成东,等.缢蛏家系生长和耐热、耐高盐性能的对比研究[J].上海海洋大学学报,2016,25(4):515-521.LI L X,DU W J,WANG C D,et al.Comparative analysis of growth and heat tolerance,salt tolerance traits among Sinonovacula constricta families[J].Journal of Shanghai Ocean University,2016,25(4):515-521.(in Chinese)
[11] CHEN Y H,DU X N,DONG Z G,et al.Heritability estimation of high salt tolerance in razor clam (Sinonovacula constricta)[J].Aquaculture,2022,559:738423.
[12] XU H Q,MO T B,LIU S,et al.Heritability estimates for ammonia resistance and growth-related traits in the razor clam Sinonovacula constricta[J].Aquaculture,2022,549:737750.
[13] CHENG D J,LI D J,YUAN Y Z,et al.Improving carbohydrate and starch accumulation in Chlorella sp.AE10 by a novel two-stage process with cell dilution[J].Biotechnology for Biofuels,2017,10:75.
[14] DE FARIAS SILVA C E,BERTUCCO A.Bioethanol from microalgae and cyanobacteria:a review and technological outlook[J].Process Biochemistry,2016,51(11):1833-1842.
[15] 董波,薛钦昭,李军.滤食性贝类摄食生理的研究进展[J].海洋科学,2000,24(7):31-34.DONG B,XUE Q Z,LI J.Research progress on feeding physiology of filter-feeding shellfish[J].Marine Sciences,2000,24(7):31-34.(in Chinese)
[16] 杨栋,韩雨婷,高葛琪,等.不同低盐驯化方式对缢蛏行为及生理的影响[J].上海海洋大学学报,2024,33(5):1120-1131.YANG D,HAN Y T,GAO G Q,et al.Behavioral and physiological responses of Sinonovacula contricta to different low-salt domestication patterns[J].Journal of Shanghai Ocean University,2024,33(5):1120-1131.(in Chinese)
[17] 杨杰青,蒋玫,李磊,等.盐度、pH对文蛤(Meretrix meretrix)滤水率和摄食率的影响[J].渔业科学进展,2016,37(6):87-93.YANG J Q,JIANG M,LI L,et al.Effects of salinity and pH on the filtration rate and ingestion rate of Meretrix meretrix[J].Progress in Fishery Sciences,2016,37(6):87-93.(in Chinese)
[18] ZHANG T,YAO J T,XU D P,et al.Gill physiological and transcriptomic response of the threatened freshwater mussel Solenaia oleivora to salinity shift[J].Comparative Biochemistry and Physiology Part D:Genomics and Proteomics,2021,40:100913.
[19] QIAN Z P,CHEN K Y,YANG L,et al.Apoptosis-inducing factor 1 mediates Vibrio splendidusinduced coelomocyte apoptosis via importin β dependent nuclear translocation in Apostichopus japonicus[J].Fish &Shellfish Immunology,2024,148:109491.
[20] LUO L,YANG L S,HUANG J H,et al.Effects of different salinity stress on the transcriptomic responses of freshwater crayfish (Procambarus clarkii,Girard,1852)[J].Biology,2024,13(7):530.
[21] ZHOU X J,CHEN Q H,CHEN L N,et al.The effect of reactive oxygen species (ROS) in immunity and WSSV infection of Scylla paramamosain[J].Fish &Shellfish Immunology,2023,141:109075.
[22] XIA H M,LI X,GAO W W,et al.Tissue repair and regeneration with endogenous stem cells[J].Nature Reviews Materials,2018,3:174-193.
[23] SATYAVATHI C,PRABHAKARA RAO Y.Inhibition of Na+,K+-ATPase in Penaeus indicus postlarvae by lead[J].Comparative Biochemistry and Physiology Part C:Pharmacology,Toxicology and Endocrinology,2000,127(1):11-22.
[24] 叶博,程之扬,彭茂潇,等.急性pH和碳酸盐碱度对缢蛏存活率、Na+/K+-ATPase活性及血淋巴吞噬能力的影响[J].水产学报,2019,43(8):1723-1732.YE B,CHENG Z Y,PENG M X,et al.Effects of pH and carbonate alkalinity on survival rate,Na+/K+-ATPase activity and phagocytic ability of the razor clam (Sinonovacula constricta)[J].Journal of Fisheries of China,2019,43(8):1723-1732.(in Chinese)
[25] 李智,彭茂潇,叶博,等.急性低盐度对缢蛏存活率、Na+/K+-ATPase活性以及血淋巴细胞吞噬能力的影响[J].上海海洋大学学报,2020,29(4):489-495.LI Z,PENG M X,YE B,et al.Effects of acute low salinity on Sinonovacula constricta survival rate,Na+/K+-ATPase activity and phagocytosis of hemocytes[J].Journal of Shanghai Ocean University,2020,29(4):489-495.(in Chinese)
[26] 裴瑞华,郑梓瑶,张诺,等.盐度胁迫对彩虹明樱蛤存活、抗氧化酶和Na+/K+-ATP酶活性的影响[J].海洋渔业,2023,45(2):162-171.PEI R H,ZHENG Z Y,ZHANG N,et al.Effects of salinity stress on survival,antioxidant enzymes and Na+/K+-ATPase activities of Moerella iridescens[J].Marine Fisheries,2023,45(2):162-171.(in Chinese)
[27] WANG Y Z,HAN Y T,WANG Y H,et al.Expression of p38MAPK and its regulation of apoptosis under high temperature stress in the razor clam Sinonovacula constricta[J].Fish &Shellfish Immunology,2022,122:288-297.
[28] AKBARIAN A,MICHIELS J,DEGROOTE J,et al.Association between heat stress and oxidative stress in poultry;mitochondrial dysfunction and dietary interventions with phytochemicals[J].Journal of Animal Science and Biotechnology,2016,7:37.
[29] DELLES R M,XIONG Y L,TRUE A D,et al.Dietary antioxidant supplementation enhances lipid and protein oxidative stability of chicken broiler meat through promotion of antioxidant enzyme activity[J].Poultry Science,2014,93(6):1561-1570.
[30] 张惠,曾霖,熊逸飞,等.盐度驯化改善大黄鱼盐度胁迫耐受性的作用机制[J].中国水产科学,2023,30(3):334-343.ZHANG H,ZENG L,XIONG Y F,et al.Mechanism of salinity acclimation in Larimichthys crocea improving tolerance to salinity stress[J].Journal of Fishery Sciences of China,2023,30(3):334-343.(in Chinese)
[31] DOMINGUEZ M,TAKEMURA A,TSUCHIYA M.Effects of changes in environmental factors on the non-specific immune response of Nile Tilapia,Oreochromis niloticus L.[J].Aquaculture Research,2005,36(4):391-397.
[32] 胡馨丹,李瑶,张小花,等.外源ATP对油菜幼苗耐寒性的影响[J].植物研究,2021,41(2):302-311.HU X D,LI Y,ZHANG X H,et al.Effect of exogenous ATP on cold tolerance of Brassica campestris seedlings[J].Bulletin of Botanical Research,2021,41(2):302-311.(in Chinese)
[33] SHEN M,CUI Y T,WANG R J,et al.Acute response of Pacific white shrimp Litopenaeus vannamei to high-salinity reductions in osmosis-,metabolism-,and immune-related enzyme activities[J].Aquaculture International,2020,28(1):31-39.
[34] RICHARD G,GU RARD F,CORPOREAU C,et al.Metabolic responses of clam Ruditapes philippinarum exposed to its pathogen Vibrio tapetis in relation to diet[J].Developmental &Comparative Immunology,2016,60:96-107.
RARD F,CORPOREAU C,et al.Metabolic responses of clam Ruditapes philippinarum exposed to its pathogen Vibrio tapetis in relation to diet[J].Developmental &Comparative Immunology,2016,60:96-107.
[35] VALIPOUR A,NEDAEI S,NOORI A,et al.Dietary Lactobacillus plantarum affected on some immune parameters,air-exposure stress response,intestinal microbiota,digestive enzyme activity and performance of narrow clawed crayfish (Astacus leptodactylus,Eschscholtz)[J].Aquaculture,2019,504:121-130.
[36] MANDUZIO H,MONSINJON T,ROCHER B,et al.Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis[J].Aquatic Toxicology,2003,64(1):73-83.
[37] BAL A,PANDA F,PATI S G,et al.Modulation of physiological oxidative stress and antioxidant status by abiotic factors especially salinity in aquatic organisms[J].Comparative Biochemistry and Physiology Part C:Toxicology &Pharmacology,2021,241:108971.
[38] HASANUZZAMAN M,BHUYAN M H M B,ZULFIQAR F,et al.Reactive oxygen species and antioxidant defense in plants under abiotic stress:revisiting the crucial role of a universal defense regulator[J].Antioxidants,2020,9(8):681.