杂交鳢是乌鳢(Channa maculate♀)和斑鳢(C.argus♂)的杂交后代,具有生长速度快(比乌鳢快20%,比斑鳢快50%)、耐低氧、抗病能力强且个体大、肉质好的特点[1],是中国特色淡水养殖鱼类之一。2023中国渔业统计年鉴数据显示,全国淡水养殖乌鳢总产量达55 万t[2],随着预制菜的发展呈递增趋势,对鱼苗的需求量也不断增加。但在鳢培苗过程中,存在苗种存活率低的情况,转食期间饲料营养不足导致肠道健康问题或生长不均匀互相残食是其主要原因[3],而开发全价适口的仔稚鱼饲料是解决问题的途径之一。
蛋白质作为水产饲料的主要营养来源,是水产动物机体的重要组分,水产动物对饲料中蛋白质的需求实际上是对氨基酸的需求[4]。研究表明,饲料蛋白质中氨基酸的比例对蛋白质的利用率起重要作用,其中蛋氨酸(Methionine)作为水生动物的限制性氨基酸之一,其含量与水产动物的生长发育息息相关[5]。作为鱼类必需氨基酸之一的蛋氨酸,不仅参与鱼体中蛋白质的合成并提供甲基供体,还是半胱氨酸和牛磺酸等物质的前体[6],若饲料中缺乏蛋氨酸将会导致鱼类生长性能降低、饲料利用率降低、机体氧化应激和系统代谢紊乱等问题[7]。已有研究表明,饲料中添加适宜的蛋氨酸可以促进鱼类的生长性能,提高饲料利用率和成活率。尼罗罗非鱼(Tilapia mossambica)幼鱼在饲料蛋氨酸水平为0.91%时获得最大的增重率,同时超氧化物歧化酶和谷胱甘肽过氧化物酶活性达到最高[8];黄颡鱼(Pelteobagrus fulvidraco)幼鱼在饲料蛋氨酸水平为1.05%时特定生长率、蛋白质效率和肥满度达到最大值,同时脏体比达到最小值[9];驼背鲈(Cromileptes altivelis)幼鱼在饲料蛋氨酸水平为1.07%时的生长性能、饲料利用率、肠道发育、蛋白质沉积率和脂肪沉积率达到最佳值[10]。目前杂交鳢对蛋氨酸的适宜需求量尚未见报道,因此,本试验中开展了饲料中不同蛋氨酸水平对杂交鳢仔稚鱼生长、生理生化、肠道消化酶活性和免疫抗氧化指标等的影响研究,以期确定杂交鳢仔稚鱼饲料中蛋氨酸的最适添加量,为杂交鳢饲料的开发提供参考依据。
1 材料与方法
1.1 材料
以进口鱼粉、大豆浓缩蛋白为主要蛋白质源,豆油、鱼油和大豆磷脂为脂肪源,面粉为主要糖源,并添加复合维生素和复合矿物质等,以褐藻酸钠作黏合剂,以丙氨酸作为等氮替代物[11],通过添加不同水平蛋氨酸(L-蛋氨酸,纯度≥95%,购自石家庄瑞远化工有限公司),配制成蛋氨酸添加量分别为0%、0.5%、1.0%、1.5%、2.0%和2.5%(实测值为分别为0.67%、1.08%、1.43%、1.78%、2.22%和2.61%)的6种等氮等脂的试验饲料,饲料蛋白质和脂肪水平等参照本试验室前期研究结果设定。将全部饲料原料分别粉碎后过198 μm筛,全部称重后混匀,微量组分采用逐级扩大法混匀,加入一定量的水,充分混匀后经制粒机(G-500)加工,55 ℃烘干后冷却至室温,饲料分别过245、350、830 μm筛网,过筛后得到粒径分别为0.3~0.425 mm和0.425~0.85 mm的颗粒饲料,并装入密封袋中于-20 ℃冰箱中保存备用。各组饲粮组成及营养水平见表1。
表1 试验饲料组成及营养水平(风干基础)
Tab 1 Component and nutrient levels of basal diets (air-dry basis) %
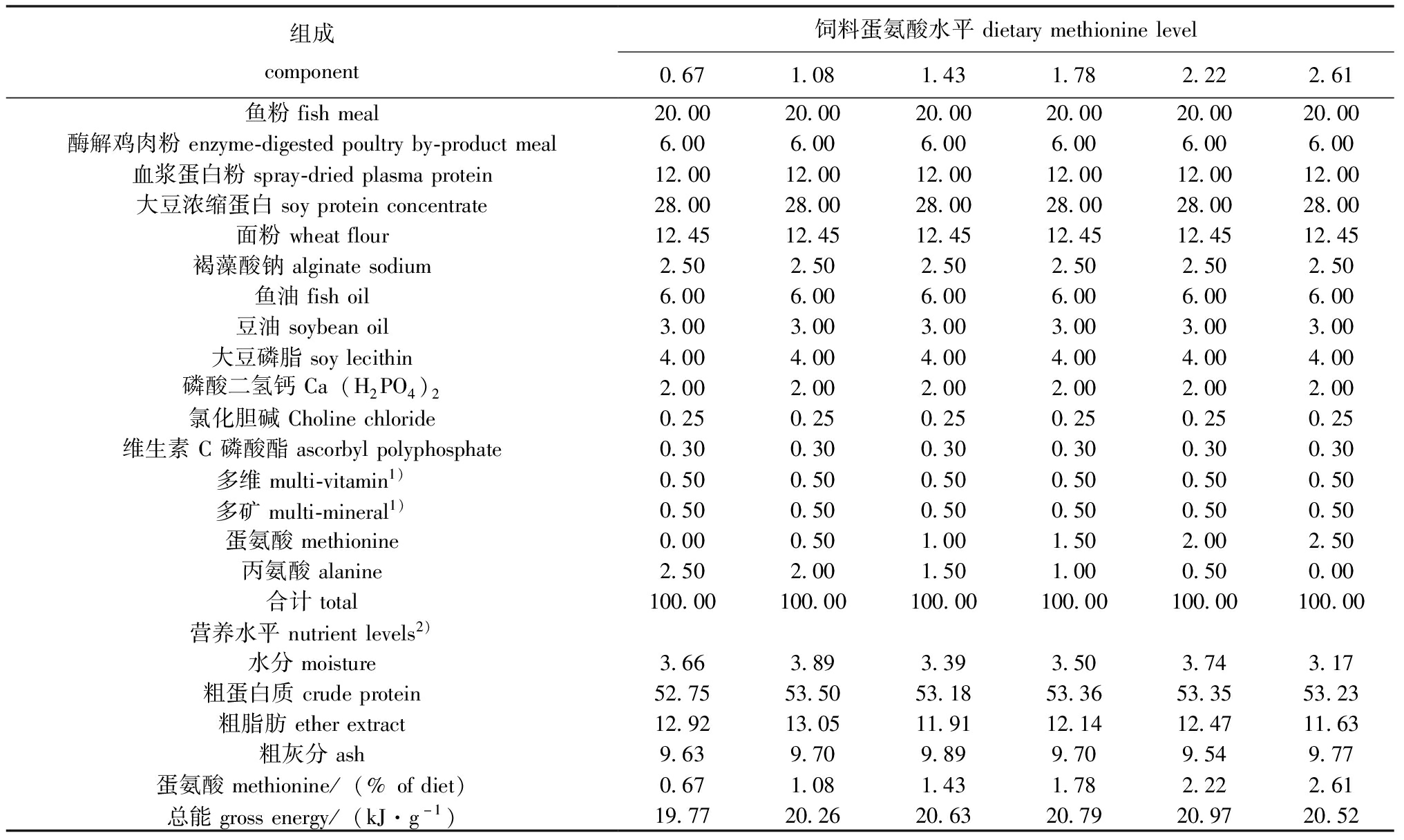
组成component饲料蛋氨酸水平dietarymethioninelevel0.671.081.431.782.222.61鱼粉fishmeal20.0020.0020.0020.0020.0020.00酶解鸡肉粉enzyme-digestedpoultryby-productmeal6.006.006.006.006.006.00血浆蛋白粉spray-driedplasmaprotein12.0012.0012.0012.0012.0012.00大豆浓缩蛋白soyproteinconcentrate28.0028.0028.0028.0028.0028.00面粉wheatflour12.4512.4512.4512.4512.4512.45褐藻酸钠alginatesodium2.502.502.502.502.502.50鱼油fishoil6.006.006.006.006.006.00豆油soybeanoil3.003.003.003.003.003.00大豆磷脂soylecithin4.004.004.004.004.004.00磷酸二氢钙Ca(H2PO4)22.002.002.002.002.002.00氯化胆碱Cholinechloride0.250.250.250.250.250.25维生素C磷酸酯ascorbylpolyphosphate0.300.300.300.300.300.30多维multi-vitamin1)0.500.500.500.500.500.50多矿multi-mineral1)0.500.500.500.500.500.50蛋氨酸methionine0.000.501.001.502.002.50丙氨酸alanine2.502.001.501.000.500.00合计total100.00100.00100.00100.00100.00100.00营养水平nutrientlevels2)水分moisture3.663.893.393.503.743.17粗蛋白质crudeprotein52.7553.5053.1853.3653.3553.23粗脂肪etherextract12.9213.0511.9112.1412.4711.63粗灰分ash9.639.709.899.709.549.77蛋氨酸methionine/(%ofdiet)0.671.081.431.782.222.61总能grossenergy/(kJ·g-1)19.7720.2620.6320.7920.9720.52
注:1)维生素预混料和矿物质预混料为每千克饲粮提供 VA 8 000 IU,VB1 4 mg,VB2 3.6 mg,VB5 40 mg,VB6 4 mg,VB12 0.02 mg,VD3 3 000 IU,VE 20 IU,VK3 2 mg,生物素0.15 mg,叶酸 1.0 mg,D-泛酸 11 mg,烟酸 10 mg,抗氧化剂100 mg,铜 10 mg,铁 80 mg,锰 80 mg,锌 75 mg,碘 0.40 mg,硒 0.30 mg;2)指实测值。
Note:1)vitamin premix and mineral premix provided the following per kg of diets:VA 8 000 IU,VB1 4 mg,VB2 3.6 mg,VB5 40 mg,VB6 4 mg,VB12 0.02 mg,VD3 3 000 IU,VE 20 IU,VK3 2 mg,biotin 0.15 mg,folic acid 1.0 mg,D-pantothenic acid 11 mg,nicotinic acid 10 mg,antioxidant 100 mg,Cu (as copper sulfate) 10 mg,Fe (as ferrous sulfate) 80 mg,Mn (as manganese sulfate) 80 mg,Zn (as zinc sulfate) 75 mg,I (as potassium iodide) 0.40 mg,Se (as sodium selenite) 0.30 mg;2)refers to the measured value.
1.2 方法
1.2.1 试验鱼与饲养管理 试验用杂交鳢鱼苗购自广东佛山顺德某养殖场。养殖于广东省农业科学院动物科学研究所水产研究室室外水泥池网箱(单个体积50 L)养殖系统中。试验开始前将杂交鳢仔稚鱼在室外循环水系统中暂养1周,期间投喂商品料(粗蛋白质45%),每天4次(08:00、11:30、15:30和19:00)。暂养结束挑选2 400尾活力强、外观健康、初始体质量为100 mg的试验鱼,随机分成6组,每组设置4个重复,每个重复放置100尾鱼。每日按时投喂并用虹吸法吸出残饵,水泥池每天排污,根据情况换少量水,表观饱食投喂,试验周期30 d。养殖期间,自然光源,养殖条件如下:水温25.0 ℃~29.5 ℃、pH 6.5~7.0、溶氧质量浓度>6 mg/L、氨氮质量浓度<0.20 mg/L和亚硝酸盐质量浓度<0.05 mg/L。
1.2.2 样品采集 养殖试验结束后禁食24 h,逐缸称重,并统计存活数。每缸随机取8尾鱼测量体长,称量体质量、内脏团质量用于计算形体指标;取7尾鱼于冰上解剖,取全肠和肝脏,-80 ℃冰箱中保存,用以检测消化酶、生化指标与抗氧化酶活性;取20尾全鱼于-80 ℃冰箱中保存用于全鱼体成分测定。动物试验由广东省农业科学院动物科学研究所实验动物伦理委员会批准,批准号2023010。
1.2.3 指标测定
分别测定存活率(SR)、增重率(WGR)、饲料系数(FCR)、特定生长率(SGR)、蛋白质效率(PER)、肥满度(CF)、脏体比(VSI),计算公式为
RSR=100×终末尾数/初始尾数,
(1)
(2)
(3)
(4)
(5)
FCF=100×体质量/体长3,
(6)
IVSI=100×内脏团质量/体质量。
(7)
1.2.4 营养成分检测 全鱼营养成分采用国标法:采用105 ℃烘箱干燥法测定水分含量(GB/T 6435—2014)、采用马弗炉550 ℃灼烧法测灰分含量(GB/T 6438—2007)、采用乙醚抽提法测定粗脂肪含量(GB/T 6433—2006)、采用凯氏定氮法测定粗蛋白质含量(GB/T 6432—2018)。采用高速氨基酸自动分析仪(Model 835—50,日本日立公司)测定样品中蛋氨酸的含量,样品在110 ℃下用浓度为6 mol/L的HCl溶液水解22 h,然后过滤水解液,定容后取2 mL减压蒸干,再加入浓度为0.02 mol/L的HCl溶液2 mL,不断振动以溶解全部氨基酸后上机进行色层分析;蛋白含量根据N×6.25计算;采用Parr1281型自动氧弹仪(Parr,Moline,IL,USA)测定总能。
1.2.5 肝脏免疫、抗氧化指标和肠道消化吸收相关指标测定 将采集到的肝脏组织混样进行抗氧化指标检测;肠道组织混样进行肠道消化酶指标检测。所有相关指标酶活性均采用试剂盒检测,试剂盒均购自建成(南京)生物工程研究所,具体测定方法参照试剂盒所附说明书。
1.3 数据处理
采用SPSS 24.0软件对数据进行单因素(ANOVA)分析,采用Duncan氏法分析组间差异显著性,试验结果用平均值±标准误(mean±S.E.)表示,显著性水平设为0.05。
2 结果与分析
2.1 饲料蛋氨酸水平对杂交鳢仔稚鱼生长性能和形体指标的影响
从表2可见,饲料中蛋氨酸水平显著影响杂交鳢仔稚鱼的WGR、SGR、FCR、PER和VSI(P<0.05),对其存活率(SR)和肥满度(CF)无显著性影响(P>0.05),表明蛋氨酸对杂交鳢仔稚鱼的生长性能有促进作用。从图1可见,当试验鱼的SGR达到峰值时对应的蛋氨酸水平为1.72%(占饲料蛋白质的3.22%)。
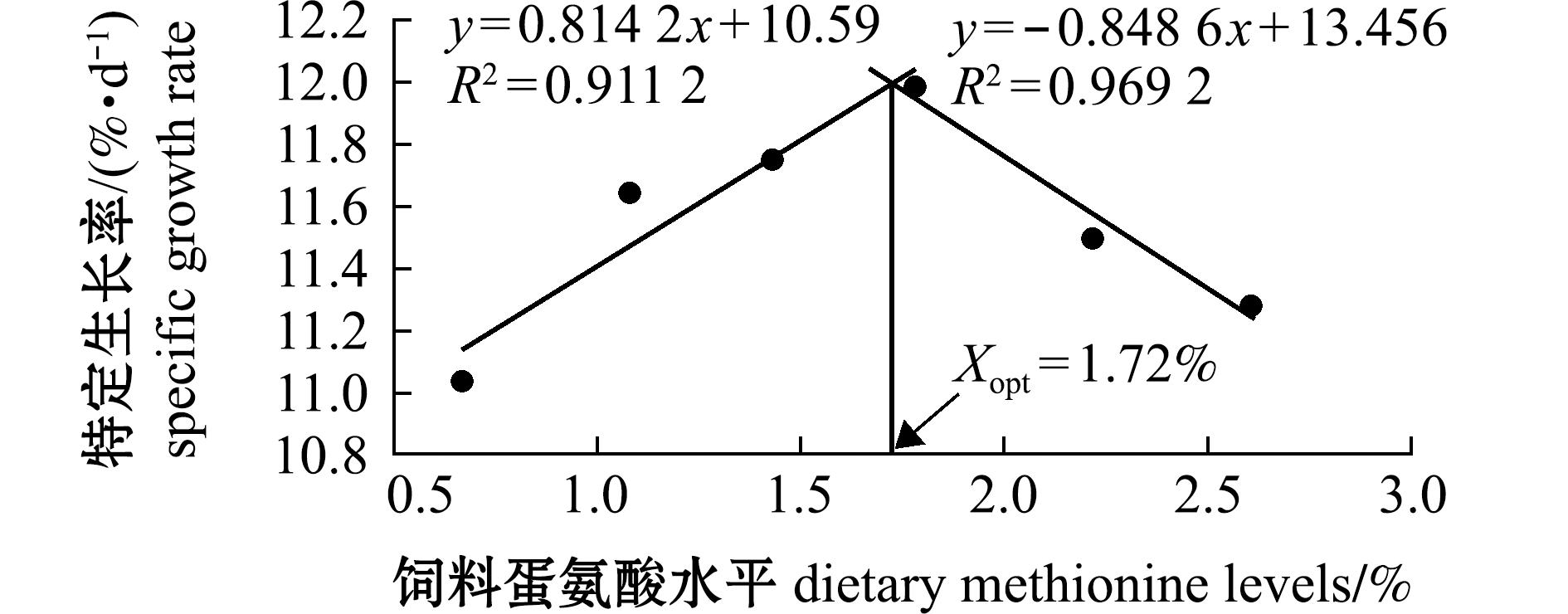
图1 饲料蛋氨酸水平对杂交鳢仔稚鱼特定生长率的回归模型
Fig.1 Regression model of dietary methionine level on specific growth rate of larval and juvenile hybrid snakehead
表2 杂交鳢仔稚鱼的生长性能和形体指标
Tab.2 Growth performance and body shape indicator of larval and juvenile hybrid snakehead
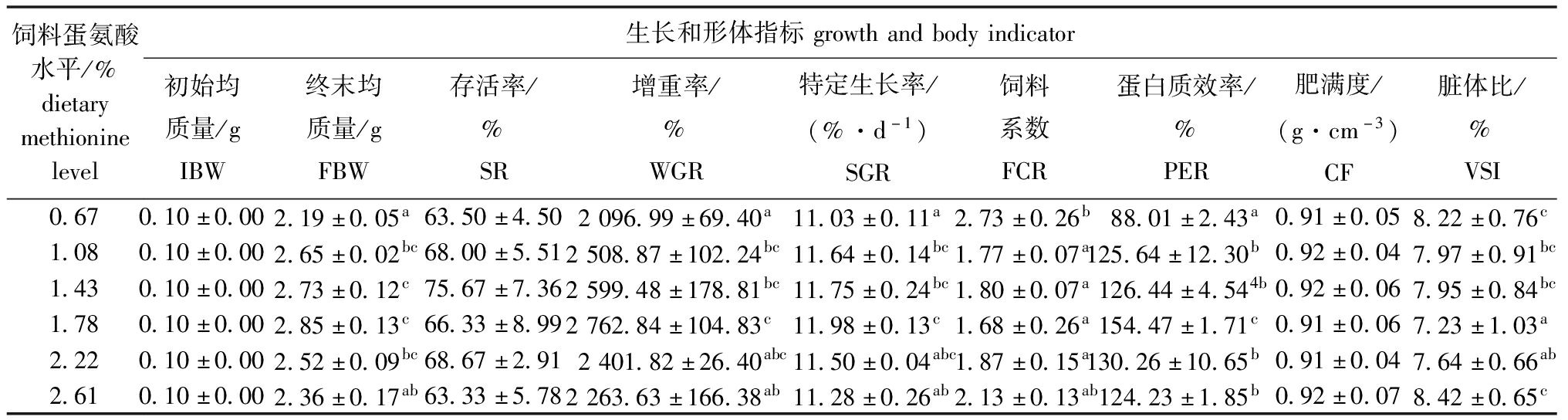
饲料蛋氨酸水平/%dietarymethioninelevel生长和形体指标growthandbodyindicator初始均质量/gIBW终末均质量/gFBW存活率/%SR增重率/%WGR特定生长率/(%·d-1)SGR饲料系数FCR蛋白质效率/%PER肥满度/(g·cm-3)CF脏体比/%VSI0.670.10±0.002.19±0.05a63.50±4.502096.99±69.40a11.03±0.11a2.73±0.26b88.01±2.43a0.91±0.058.22±0.76c1.080.10±0.002.65±0.02bc68.00±5.512508.87±102.24bc11.64±0.14bc1.77±0.07a125.64±12.30b0.92±0.047.97±0.91bc1.430.10±0.002.73±0.12c75.67±7.362599.48±178.81bc11.75±0.24bc1.80±0.07a126.44±4.544b0.92±0.067.95±0.84bc1.780.10±0.002.85±0.13c66.33±8.992762.84±104.83c11.98±0.13c1.68±0.26a154.47±1.71c0.91±0.067.23±1.03a2.220.10±0.002.52±0.09bc68.67±2.912401.82±26.40abc11.50±0.04abc1.87±0.15a130.26±10.65b0.91±0.047.64±0.66ab2.610.10±0.002.36±0.17ab63.33±5.782263.63±166.38ab11.28±0.26ab2.13±0.13ab124.23±1.85b0.92±0.078.42±0.65c
注:同行中标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05),下同。
Note:The means with different letters within the same line are significantly different in the groups at the 0.05 probability level,and the means with the same letter within the same line are not significant differences,et sequentia.
2.2 饲料蛋氨酸水平对杂交鳢仔稚鱼体成分及肝脏生化指标的影响
从表3和表4可见,饲料蛋氨酸水平对杂交鳢仔稚鱼体脂肪、水分和灰分的含量有显著性影响(P<0.05),对肝脏中TG和TC含量及AST活性也有显著影响(P<0.05)。其中粗脂肪和灰分含量总体呈先升后降趋势,且均在1.08%试验组达到最大值,分别显著大于0.67%和2.61%试验组(P<0.05)。水分含量则总体呈先降后升的趋势,在1.08%试验组时达到最低值;对其粗蛋白和葡萄糖(GLU)含量及谷丙转氨酶(ALT)活性均无显著性影响(P>0.05)。从图2可见,试验鱼肝脏谷草转氨酶活性达到最高时蛋氨酸水平为1.80%。
表3 杂交鳢仔稚鱼的鱼体成分(湿物质)
Tab.3 Components of larval and juvenile hybrid snakehead(wet matter) %
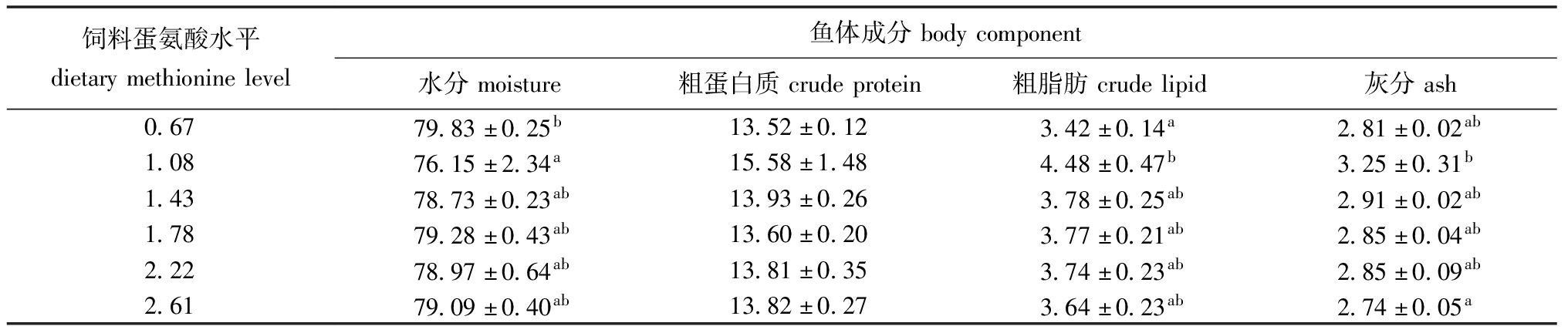
饲料蛋氨酸水平dietarymethioninelevel鱼体成分bodycomponent水分moisture粗蛋白质crudeprotein粗脂肪crudelipid灰分ash0.6779.83±0.25b13.52±0.123.42±0.14a2.81±0.02ab1.0876.15±2.34a15.58±1.484.48±0.47b3.25±0.31b1.4378.73±0.23ab13.93±0.263.78±0.25ab2.91±0.02ab1.7879.28±0.43ab13.60±0.203.77±0.21ab2.85±0.04ab2.2278.97±0.64ab13.81±0.353.74±0.23ab2.85±0.09ab2.6179.09±0.40ab13.82±0.273.64±0.23ab2.74±0.05a
表4 杂交鳢仔稚鱼肝脏生化指标
Tab.4 Liver biochemical indicators of larval and juvenile hybrid snakehead
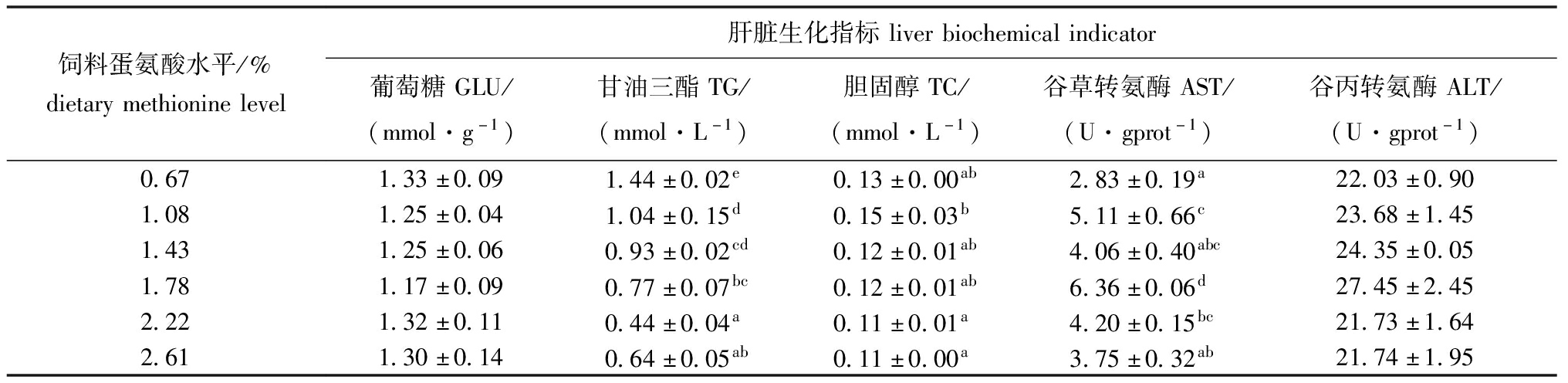
饲料蛋氨酸水平/%dietarymethioninelevel肝脏生化指标liverbiochemicalindicator葡萄糖GLU/(mmol·g-1)甘油三酯TG/(mmol·L-1)胆固醇TC/(mmol·L-1)谷草转氨酶AST/(U·gprot-1)谷丙转氨酶ALT/(U·gprot-1)0.671.33±0.091.44±0.02e0.13±0.00ab2.83±0.19a22.03±0.901.081.25±0.041.04±0.15d0.15±0.03b5.11±0.66c23.68±1.451.431.25±0.060.93±0.02cd0.12±0.01ab4.06±0.40abc24.35±0.051.781.17±0.090.77±0.07bc0.12±0.01ab6.36±0.06d27.45±2.452.221.32±0.110.44±0.04a0.11±0.01a4.20±0.15bc21.73±1.642.611.30±0.140.64±0.05ab0.11±0.00a3.75±0.32ab21.74±1.95
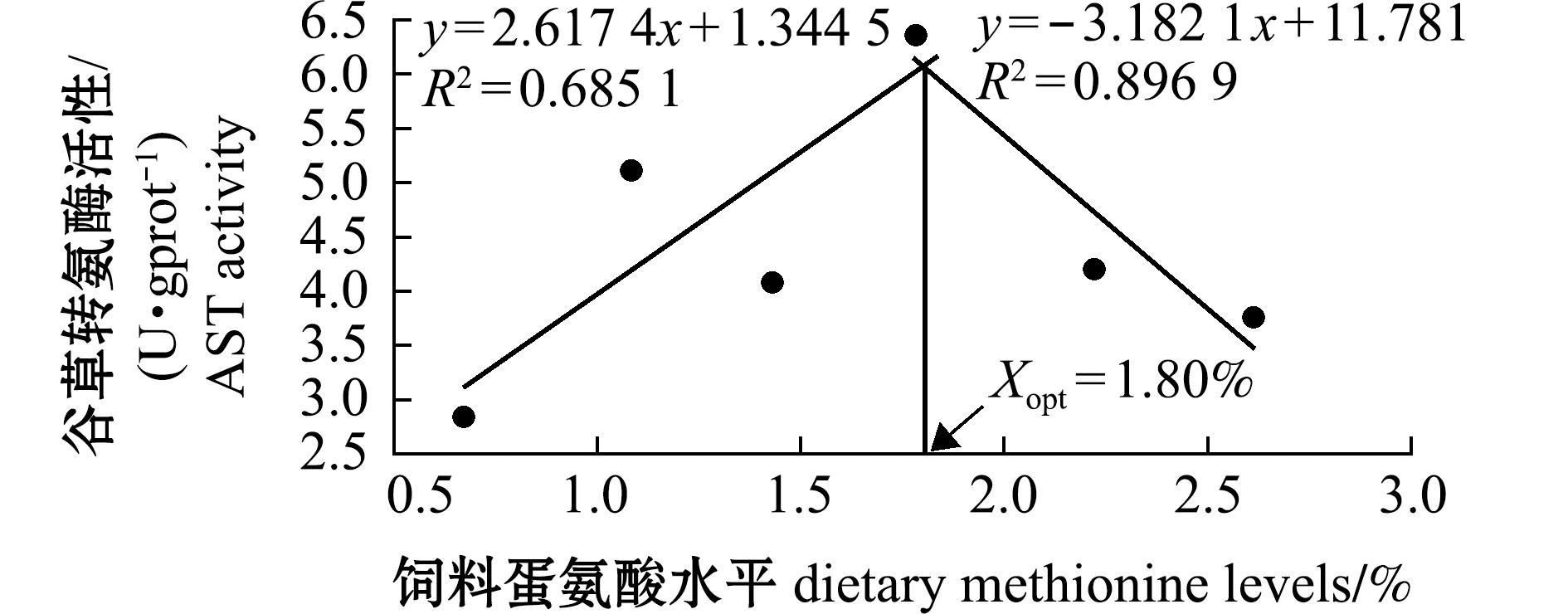
图2 饲料蛋氨酸水平对杂交鳢仔稚鱼谷草转氨酶的回归模型
Fig.2 Regression model of dietary methionine level on AST of larval and juvenile hybrid snakehead
2.3 饲料蛋氨酸水平对杂交鳢仔稚鱼肠道消化酶活性的影响
从表5可见,饲料蛋氨酸水平显著影响杂交鳢仔稚鱼的肠道胰蛋白酶和脂肪酶活性(P<0.05)。其中胰蛋白酶和脂肪酶活性均呈现先升后降的趋势,1.78%试验组达到最大值,显著高于0.67%试验组(P<0.05);对淀粉酶无显著性影响(P>0.05),表明适宜的蛋氨酸水平能够改善杂交鳢仔稚鱼的肠道消化功能。从图3和图4可见,当胰蛋白酶活性达到最高时对应的蛋氨酸水平为1.75%;当脂肪酶活性达到最高时对应的蛋氨酸水平为1.73%。
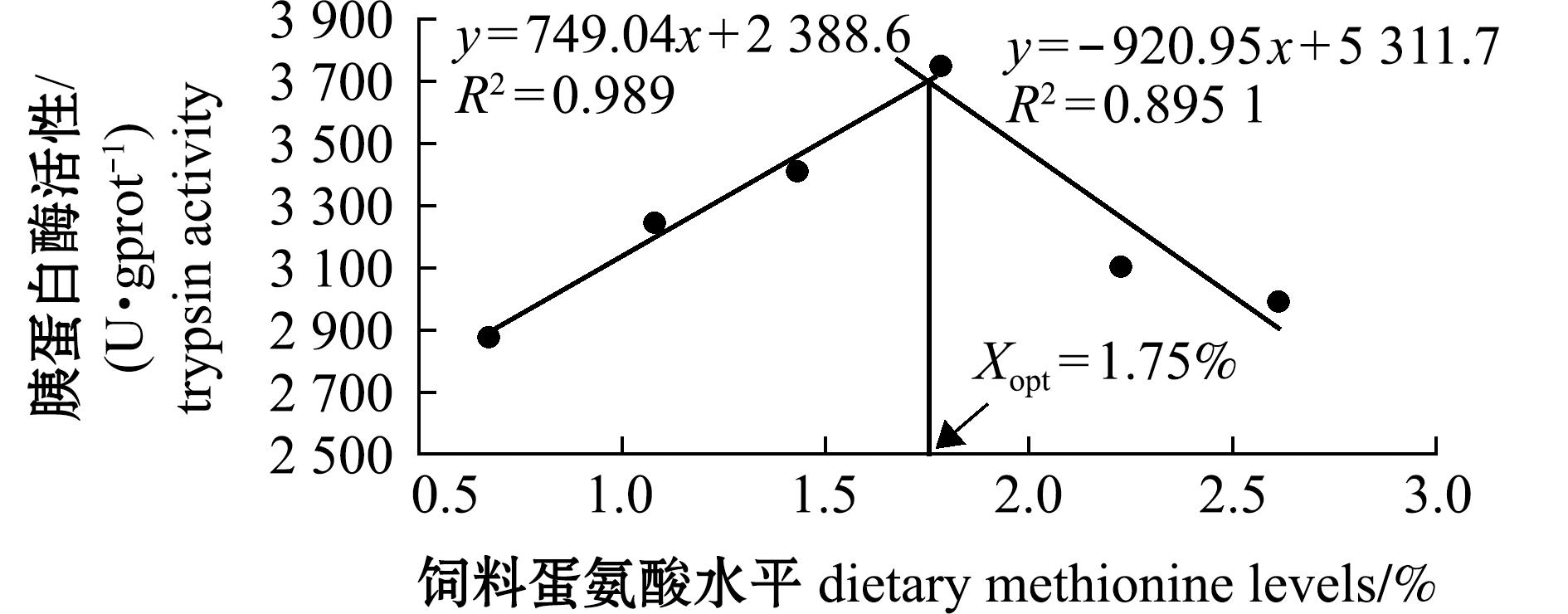
图3 饲料蛋氨酸水平对杂交鳢仔稚鱼肠道胰蛋白酶活性的回归模型
Fig.3 Regression model of dietary methionine levels on intestinal trypsin activity of larval and juvenile hybrid snakehead
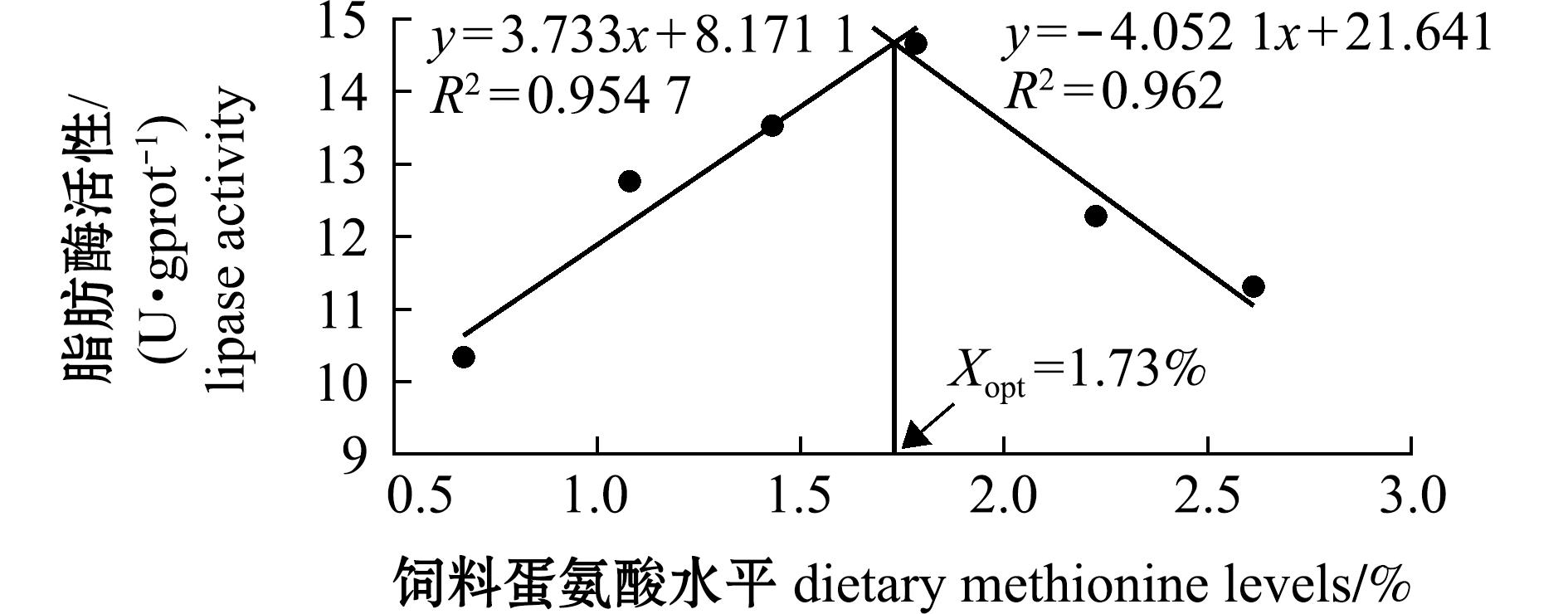
图4 饲料蛋氨酸水平对杂交鳢仔稚鱼肠道脂肪酶活性的回归模型
Fig.4 Regression model of dietary methionine levels on intestinal lipase activity of larval and juvenile hybrid snakehead
表5 杂交鳢仔稚鱼肠道消化酶活性
Tab.5 Inteintestinal digestive enzyme activities of larval and juvenile hybrid snakehead
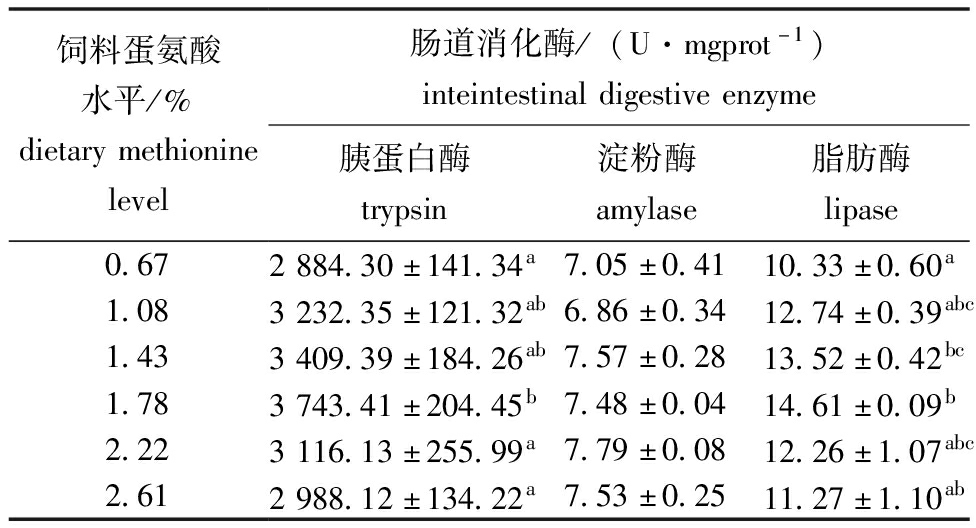
饲料蛋氨酸水平/%dietarymethioninelevel肠道消化酶/(U·mgprot-1)inteintestinaldigestiveenzyme胰蛋白酶trypsin淀粉酶amylase脂肪酶lipase0.672884.30±141.34a7.05±0.4110.33±0.60a1.083232.35±121.32ab6.86±0.3412.74±0.39abc1.433409.39±184.26ab7.57±0.2813.52±0.42bc1.783743.41±204.45b7.48±0.0414.61±0.09b2.223116.13±255.99a7.79±0.0812.26±1.07abc2.612988.12±134.22a7.53±0.2511.27±1.10ab
2.4 饲料蛋氨酸水平对杂交鳢仔稚鱼肝脏抗氧化指标的影响
从表6可见,饲料蛋氨酸水平显著影响杂交鳢仔稚鱼的肝脏T-SOD、MDA和GSH-Px(P<0.05),对过氧化氢酶(CAT)活性无显著性影响(P>0.05)。其中T-SOD总体呈先升后降的趋势,表明适宜的蛋氨酸水平能够提高杂交鳢仔稚鱼的抗氧化能力。
表6 杂交鳢仔稚鱼肝脏的抗氧化指标
Tab.6 Antioxidant indicators of liver of larval and juvenile hybrid snakehead
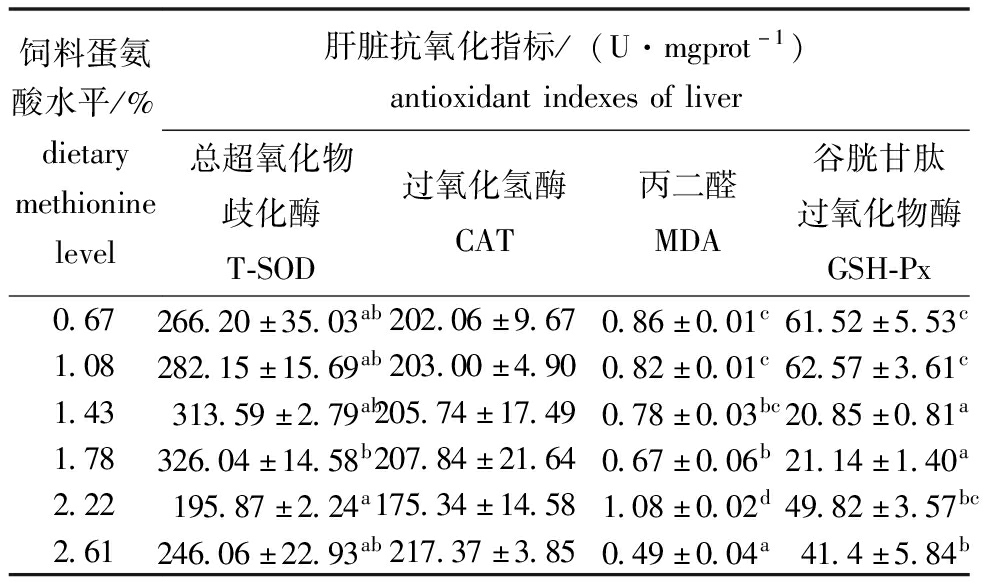
饲料蛋氨酸水平/%dietarymethioninelevel肝脏抗氧化指标/(U·mgprot-1)antioxidantindexesofliver总超氧化物歧化酶T-SOD过氧化氢酶CAT丙二醛MDA谷胱甘肽过氧化物酶GSH-Px0.67266.20±35.03ab202.06±9.670.86±0.01c61.52±5.53c1.08282.15±15.69ab203.00±4.900.82±0.01c62.57±3.61c1.43313.59±2.79ab205.74±17.490.78±0.03bc20.85±0.81a1.78326.04±14.58b207.84±21.640.67±0.06b21.14±1.40a2.22195.87±2.24a175.34±14.581.08±0.02d49.82±3.57bc2.61246.06±22.93ab217.37±3.850.49±0.04a41.4±5.84b
3 讨论
3.1 蛋氨酸水平对杂交鳢仔稚鱼生长性能的影响
在蛋氨酸水平为1.38%时,红鳍东方鲀幼鱼的SGR达到峰值[12];在蛋氨酸水平为0.90%时,松浦镜鲤幼鱼获得最大的WGR[13];在蛋氨酸水平为0.85%时,团头鲂幼鱼的SGR达到最大[14],上述试验鱼所需最适蛋氨酸水平均低于本试验杂交鳢仔稚鱼对蛋氨酸的最适水平1.72%,原因可能是不同食性的鱼类对蛋氨酸的适宜需求量不同,肉食性鱼类红鳍东方鲀幼鱼对蛋氨酸的需求量为1.38%[12],低于杂交鳢仔稚鱼对蛋氨酸的需求,说明仔稚鱼相较于幼鱼可能需要更多的蛋氨酸用于自身生长代谢。蛋氨酸可以通过调节生长激素(GH)和胰岛素生长因子1(IGF-1)的基因表达,进而调节蛋白质代谢[15],其中IGF1不仅可以通过内分泌、旁分泌和自分泌信号传导促进许多系统中的细胞增殖和分化,还能与细胞膜中的受体结合,通过PI3K/Akt途径激活TOR途径相关基因的表达,从而影响鱼类的蛋白质沉积和生长,当饲料中蛋氨酸缺乏时会导致IGF1基因表达量下调,从而抑制鱼体生长[16];当饲料中蛋氨酸过量时会导致其氧化成酮,危害鱼体健康[17]。本研究结果表明,投喂缺乏或过量的蛋氨酸水平饲料下,杂交鳢仔稚鱼都表现出较高的饵料系数和较低的蛋白质效率,说明适宜水平的蛋氨酸有利于鱼对饲料的吸收和利用,这与军曹鱼幼鱼的研究结果一致[18]。
3.2 蛋氨酸水平对杂交鳢仔稚鱼形体指标的影响
形体指标是评价鱼体生长和健康状况的重要指标,VSI和CF过高,机体可能出现炎症或者脂肪堆积,造成肝脏代谢功能损伤并影响养分的消化吸收,进而降低饲料利用率[19]。若饲料中蛋氨酸的添加量不足,将会限制鱼体内蛋白质的合成,会使本应该用来合成肌肉蛋白的氨基酸转换为糖原储存在体内最终导致鱼体脏体比增加[20];随着饲料蛋氨酸添加至适宜水平,蛋白质合成不受限制,减少了糖原在肝脏的累积,导致脏体比下降,这与大口黑鲈(Micropterus salmoides)的研究结果相似[21];当饲料中添加过量的蛋氨酸时可能会上调mTOR途径,使TOR和S6K1在肝脏中进行高表达,促进脂质合成,从而导致VSI升高[22]。在异育银鲫(Carassiusauratus gibelio)幼鱼的研究中发现,蛋氨酸的缺乏与过量,都会导致试验鱼的血氨含量显著提高[23],说明缺乏或者过量的蛋氨酸都会影响幼鱼的肝功能健康;在大西洋鲑(Salmo salar)的研究中发现,蛋氨酸的限制使鱼体脂肪酸合成酶活性增加和肝脏三酰甘油积累,从而导致脏体比增加[24];过量的蛋氨酸则推测是其代谢产生的S-腺苷甲硫氨酸在肝脏积累使肝脏受损,继而导致肝脏炎症发生,增大了脏体比。蛋氨酸能够参与鱼体内的脂肪调控过程,促进鱼体内脂肪的沉积,在大菱鲆(Scophthalmus maximus)的研究中发现,随着蛋氨酸水平的提高,鱼体粗脂肪出现上升的趋势[25],与本试验结果一致;在斜带石斑鱼(Epinephelus coioides)的研究中发现,随着蛋氨酸水平的提升,鱼体粗脂肪显著下降[26],与本试验结果相反;在军曹鱼的研究中发现,蛋氨酸水平对鱼体粗脂肪无影响[27]。由此可见,蛋氨酸对各鱼类体脂肪的影响不尽相同,其中原因有待进一步研究。
3.3 蛋氨酸水平对杂交鳢仔稚鱼生化指标和消化吸收能力的影响
肝脏生化指标是反映机体代谢机能、健康状况的重要指标。饲料中添加适宜水平蛋氨酸能提高水产动物对碳水化合物的代谢水平,从而降低鱼体的体脂水平[28]。肝胰脏ALT和AST是氨基酸代谢的关键酶,能够反映氨基酸的代谢强度。随着蛋氨酸水平的提高,洛氏鱥(Phoxinus lagowskii)肝脏中AST和ALT的活性呈先升后降趋势,在蛋氨酸水平为1.62%时达到最大值[29],低于本试验水平,可能是因为不同规格鱼类对蛋氨酸的最适需求量存在差异,稚鱼体内新陈代谢旺盛需要更多的氨基酸用于蛋白质的合成;黄颡鱼幼鱼肝脏中AST和ALT的活性呈先升后降的趋势,在蛋氨酸水平为1.70%时达到最佳[30],略低于杂交鳢仔稚鱼对蛋氨酸的最适需求量,可能是本试验饲料在水中浸泡导致添加的晶体氨基酸溶解流失,从而导致氨基酸分解代谢过多,造成蛋氨酸需求量的研究结果过高。这些试验说明适宜水平的蛋氨酸可以改善鱼体肝脏中氨基酸的利用与代谢,使机体氨基酸水平达到平衡状态,从而促进鱼类的生长发育。
肠道是鱼类消化和吸收营养物质的主要场所,其中消化酶活力是评价鱼类消化机能的重要指标,鱼类消化酶主要包括由消化腺和消化系统分泌的胰蛋白酶、淀粉酶和脂肪酶。研究表明,蛋氨酸缺乏可导致消化酶活性降低,日粮中添加包被蛋氨酸可显著提高肠道消化酶活性,且肠道消化酶活性的变化趋势与WGR变化相似[31]。本试验中肠道胰蛋白酶随蛋氨酸水平升高呈现先升后降的趋势,经回归模型分析,蛋氨酸水平为1.75%时胰蛋白酶活性最高,与幼建鲤(Cyprinus carpio)肠道胰蛋白酶活性随蛋氨酸水平提高呈先升后降趋势,在蛋氨酸水平为1.00%时,胰蛋白酶活性达到最高[32]的研究结果一致。脂肪是维持鱼类正常生长、发育和繁殖的必需营养物质,在鱼类生命活动中发挥着重要的作用。蛋氨酸能够通过介导TOR途径上调或下调SREBP的表达以促进或抑制甘油三酯和脂肪酸的合成[33]。本研究中,脂肪酶含量随着蛋氨酸水平的提高呈先升后降趋势且肝脏甘油三酯含量下降,说明可能在蛋氨酸水平适宜时上调了SREBP的表达,另外,大菱鲆[7]在蛋氨酸缺乏时,TOR活性降低,SREBP的表达水平下调也证实了这点。说明适宜的蛋氨酸水平能够增强鱼体的消化能力,促进对营养物质的吸收,进而促进鱼体生长,这与本研究中生长性能研究结果相一致。
3.4 蛋氨酸水平对杂交鳢稚鱼抗氧化指标的影响
蛋氨酸是细胞抗氧化系统的重要组成部分,保护细胞免受氧化损伤[34]。在鱼类的研究中,蛋氨酸的抗氧化作用已经得到证实[35]。蛋氨酸能够通过上调NF-E2相关因子介导的抗氧化因子和酶活性以及NF-κB介导的抗炎因子,同时下调促炎因子,从而提高试验动物的抗氧化能力[36]。鱼类处于有氧的环境中易产生活性氧(ROS),当ROS的产生与消除的稳态遭到破坏时会引起机体的氧化损伤,此时抗氧化防御系统就会通过相应抗氧化酶的作用以减轻损伤[37]。抗氧化酶如T-SOD、CAT和GSH-Px均可以清除氧自由基以减少氧化损伤并在免疫系统中发挥关键作用,而MDA是ROS引起脂质过氧化和氧化损伤的重要指标,其含量反映鱼类的抗氧化状况[38]。本试验中最佳蛋氨酸水平可减少肝脏中MDA的积累,提高SOD活性,可能有助于抑制杂交鳢仔稚鱼组织中的脂质过氧化,与幼建鲤[39]的研究结果相似。
4 结论
1)在饲料中添加适宜的蛋氨酸可以提高杂交鳢仔稚鱼的WGR和SGR,同时降低FCR,说明适宜的蛋氨酸添加量有利于杂交鳢仔稚鱼的生长。
2)在饲料中添加适宜的蛋氨酸可以显著提高杂交鳢仔稚鱼的肠道胰蛋白酶和脂肪酶活性,表明适宜的蛋氨酸添加量能够增强杂交鳢仔稚鱼对营养物质的消化吸收能力。
3)在饲料中添加适宜的蛋氨酸可以显著提高杂交鳢仔稚鱼的肝脏T-SOD活性,同时降低MDA含量,表明适宜的蛋氨酸添加量能够提升杂交鳢仔稚鱼的肝脏抗氧化能力。
4)本试验条件下,对杂交鳢仔稚鱼最适的蛋氨酸需要量为饲料干质量的1.72%(占饲料蛋白质的3.22%)。
[1] 李培佳,陈晓瑛,赵红霞,等.精氨酸对杂交鳢生长性能、体组成、血浆生化指标及抗氧化能力的影响[J].动物营养学报,2022,34(0):1820-1830.LI P J,CHEN X Y,ZHAO H X,et al.Effects of arginine on growth performance,body composition,plasma biochemical indexes and antioxidant capacity of Hybrid Snakehead (Channa maculata♀×Channa argus♂)[J].Chinese Journal of Animal Nutrition,2022,34(3):1820-1830.(in Chinese)
[2] 农业农村部渔业渔政管理局,全国水产技术推广总站,中国水产学会.2023中国渔业统计年鉴[M].北京:中国农业出版社,2023.Bureau of Fisheries,Ministry of Agriculture and Rural Affairs,National Fisheries Technology Extension Center,China Society of Fisheries.2023 China Fishery statistical yearbook[M].Beijing:China Agriculture Press,2023.(in Chinese)
[3] 郑晶,蒋余,吴晓清,等.4种诱食剂对杂交鳢生长和血清生化指标的影响[J].动物营养学报,2016,28(8):2497-2503.ZHENG J,JIANG Y,WU X Q,et al.Effects of four feeding attractants on growth and serum biochemical indices of hybrid snakehead[J].Chinese Journal of Animal Nutrition,2016,28(8):2497-2503.(in Chinese)
[4] 毛华东,王娜,隋超,等.饲料蛋氨酸水平对绿鳍马面鲀幼鱼生长性能的影响[J].饲料工业,2024,45(6):81-89.MAO H D,WANG N,SUI C,et al.Effects of dietary methionine on growth performance of juvenile Green-Finned Filefish(Thamnaconus septentrionalis)[J].Feed Industry,2024,45(6):81-89.(in Chinese)
[5] HOSEINI S M,HOSSEINI S A,ESKANDARI S,et al.Effect of dietary taurine and methionine supplementation on growth performance,body composition,taurine retention and lipid status of Persian sturgeon,Acipenser persicus(Borodin,1897),fed with plant-based diet[J].Aquaculture Nutrition,2018,24(1):324-331.
[6] WANG L,GAO C,WANG B,et al.Methionine in fish health and nutrition:Potential mechanisms,affecting factors,and future perspectives[J].Aquaculture,2023,568:739310.
[7] JIANG H W,BIAN F Y,ZHOU H H,et al.Nutrient sensing and metabolic changes after methionine deprivation in primary muscle cells of turbot (Scophthalmus maximus L.)[J].The Journal of Nutritional Biochemistry,2017,50:74-82.
[8] HE J Y,LONG W Q,HAN B,et al.Effect of dietaryl-methionine concentrations on growth performance,serum immune and antioxidative responses of juvenile Nile Tilapia,Oreochromis niloticus[J].Aquaculture Research,2017,48(2):665-674.
[9] CHEN Y,CAO J M,HUANG Y H,et al.The dietary L-methionine requirement of the juvenile yellow catfish Pelteobagrus fulvidraco[J].Israeli Journal of Aquaculture-Bamidgeh,2014,66:1-9.
[10] 穆伟.驼背鲈幼鱼最适精氨酸、赖氨酸和蛋氨酸需求量研究[D].海口:海南大学,2020.MU W.Study on the optimum requirement of arginine,lysine and methionine for Juvenile humpback bass[D].Haikou:Hainan University,2020.(in Chinese)
[11] 张莹,张家松,高春山,等.饲料赖氨酸水平对鳜幼鱼生长、饲料利用及生化组成的影响[J].中国饲料,2022,(16):67-71.ZHANG Y,ZHANG J X,GAO C S,et al.Effect of dietary lysine level on the growth,feed utilization and biochemical composition of juveniles Siniperca chuatsi[J].China Feed,2022,(16):67-71.(in Chinese)
[12] 张庆功.红鳍东方鲀幼鱼赖氨酸、蛋氨酸、精氨酸营养生理研究[D].上海:上海海洋大学,2019.ZHANG Q G.Study on nutritional physiology of lysine,methionine and arginine in juvenile Takifugu rubripes[D].Shanghai:Shanghai Ocean University,2019.(in Chinese)
[13] 程龙.蛋氨酸对松浦镜鲤生长性能、消化吸收、抗氧化能力和肌肉合成通路基因表达的影响[D].上海:上海海洋大学,2020.CHENG L.Effects of methionine on growth performance,digestion and absorption,antioxidant capacity and gene expression of muscle synthesis pathway in Songpu mirror carp[D].Shanghai:Shanghai Ocean University,2020.(in Chinese)
[14] 廖英杰.团头鲂幼鱼对蛋氨酸、赖氨酸和精氨酸需要量的研究[D].南京:南京农业大学,2014.LIAO Y J.Study on the requirement of methionine,lysine and arginine for juvenile Megalobrama amblycephala[D].Nanjing:Nanjing Agricultural University,2014.(in Chinese)
[15] ROLLAND M,DALSGAARD J,HOLM J,et al.Dietary methionine level affects growth performance and hepatic gene expression of GH-IGF system and protein turnover regulators in rainbow trout (Oncorhynchus mykiss) fed plant protein-based diets[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2015,181:33-41.
[16] CHEN J L,ALBERTS I,LI X H.Dysregulation of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum disorders[J].International Journal of Developmental Neuroscience,2014,35:35-41.
[17] SAMADDAR A,KAVIRAJ A,SAHA S.Utilization of fermented animal by-product blend as fishmeal replacer in the diet of Labeo rohita[J].Aquaculture Reports,2015,1:28-36.
[18] CHI S Y,HE Y F,ZHU Y,et al.Dietary methionine affects growth and the expression of key genes involved in hepatic lipogenesis and glucose metabolism in cobia (Rachycentron canadum)[J].Aquaculture Nutrition,2020,26(1):123-133.
[19] 刘建高,钟雷,袁俊光,等.不同木薯淀粉水平对全养殖周期大口黑鲈生长性能、饲料利用和形体指标的影响[J].饲料工业,2023,44(2):76-80.LIU J G,ZHONG L,YUAN J G,et al.Effects of cassava starch supplementation level on growth performance,feed utilization,morphometric parameters of largemouth Bass (Micropterus salmoides) for a long-term culture[J].Feed Industry,2023,44(2):76-80.(in Chinese)
[20] YANG S D,LIU F G,LIOU C H.Assessment of dietary lysine requirement for silver perch (Bidyanus bidyanus) juveniles[J].Aquaculture,2011,312(1/2/3/4):102-108.
[21] 陈乃松,马建忠,周恒永,等.大口黑鲈对饲料中蛋氨酸需求量的评定[J].水产学报,2010,34(8):1244-1253.CHEN N S,MA J Z,ZHOU H Y,et al.Assessment of dietary methionine requirement in largemouth bass,Micropterus salmoides[J].Journal of Fisheries of China,2010,34(8):1244-1253.(in Chinese)
[22] TAN V P,MIYAMOTO S.Nutrient-sensing mTORC1:integration of metabolic and autophagic signals[J].Journal of Molecular and Cellular Cardiology,2016,95:31-41.
[23] REN M,LIANG H,HE J,et al.Effects of DL-methionine supplementation on the success of fish meal replacement by plant proteins in practical diets for juvenile gibel carp (Carassius auratus gibelio)[J].Aquaculture Nutrition,2017,23(5):934-941.
[24] ESPE M,RATHORE R M,DU Z Y,et al.Methionine limitation results in increased hepatic FAS activity,higher liver 18:1 to 18:0 fatty acid ratio and hepatic TAG accumulation in Atlantic salmon,Salmo salar[J].Amino Acids,2010,39(2):449-460.
[25] MA R,HOU H P,MAI K S,et al.Comparative study on the effects of L-methionine or 2-hydroxy-4-(methylthio) butanoic acid as dietary methionine source on growth performance and anti-oxidative responses of turbot (Psetta maxima)[J].Aquaculture,2013,412:136-143.
[26] LUO Z,LIU Y J,MAI K S,et al.Dietary l-methionine requirement of juvenile grouper Epinephelus coioides at a constant dietary cystine level[J].Aquaculture,2005,249(1/2/3/4):409-418.
[27] ZHOU Q C,WU Z H,TAN B P,et al.Optimal dietary methionine requirement for Juvenile Cobia (Rachycentron canadum)[J].Aquaculture,2006,258(1/2/3/4):551-557.
[28] 黄文庆,王梦华,李国立,等.发酵黑水虻对大口黑鲈生长性能、体成分、抗氧化指标以及肠道组织结构的影响[J].饲料工业,2024(12),20-28.HUANG W Q,WANG M H,LI G L,et al.Effects of fermented black soldier fly larvae(Hermetia illucens) on growth performance,body composition,antioxidant indexes and intestinal structure of Micropterus salmoides[J].Feed Industry,2024(12),20-28.(in Chinese)
[29] 段晶,吴莉芳,王婧瑶,等.蛋氨酸水平对洛氏鱥生长及消化酶和蛋白质代谢酶活力的影响[J].西北农林科技大学学报(自然科学版),2019,47(7):23-31.DUAN J,WU L F,WANG J Y,et al.Effects of methionine level on growth performance and activities of digestive enzymes and protein metabolism enzymes of Rhynchocypris lagowskii Dybowski[J].Journal of Northwest A &F University (Natural Science Edition),2019,47(7):23-31.(in Chinese)
[30] 王香丽,麦康森,徐玮,等.蛋氨酸对瓦氏黄颡鱼幼鱼肝脏及血浆中谷草转氨酶和谷丙转氨酶活力的影响[J].中国海洋大学学报(自然科学版),2015,45(9):49-53.WANG X L,MAI K S,XU W,et al.Influence of dietary methionine on the activity of liver and plasma Glutamic-Pyruvic and glutamic oxalacetic transaminases of juvenile darkbarbel catfish(Pelteobagrus vachelli)[J].Periodical of Ocean University of China(Natural Science Edition),2015,45(9):49-53.(in Chinese)
[31] DU Y Y,LIN X W,SHAO X P,et al.Effects of supplementing coated methionine in a high plant-protein diet on growth,antioxidant capacity,digestive enzymes activity and expression of TOR signaling pathway associated genes in gibel carp,Carassius auratus gibelio[J].Frontiers in Immunology,2024,15:1319698.
[32] 彭艳,唐凌,帅柯,等.蛋氨酸对幼建鲤生长及消化吸收功能的影响[J].中国畜牧杂志,2009,45(13):33-38.PENG Y,TANG L,SHUAI K,et al.Effect of methionine on digestive function of juvenile Jian carp(Cyprinus carpio var.Jian)[J].Chinese Journal of Animal Science,2009,45(13):33-38.(in Chinese)
[33] HORTON J D,SHAH N A,WARRINGTON J A,et al.Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes[J].Proceedings of the National Academy of Sciences of the United States of America,2003,100(21):12027-12032.
[34] COLOVIC M B,VASIC V M,DJURIC D M,et al.Sulphur-containing amino acids:protective role against free radicals and heavy metals[J].Current Medicinal Chemistry,2018,25(3):324-335.
[35] FENG L,XIAO W W,LIU Y,et al.Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var.Jian)[J].Aquaculture Nutrition,2011,17(6):595-604.
[36] YU H,MASAGOUNDER K,LIANG H L,et al.DL-methionyl-DL-methionine/DL-methionine supplementation alleviated the adverse effects of dietary low fishmeal levels on growth and intestinal health of Micropterus salmoides[J].Antioxidants,2024,13(3):359.
[37] BILLER J D,TAKAHASHI L S.Oxidative stress and fish immune system:phagocytosis and leukocyte respiratory burst activity[J].Anais Da Academia Brasileira De Ciencias,2018,90(4):3403-3414.
[38] LIN S M,PAN Y,LUO L,et al.Effects of dietary β-1,3-glucan,chitosan or raffinose on the growth,innate immunity and resistance of koi (Cyprinus carpio koi)[J].Fish &Shellfish Immunology,2011,31(6):788-794.
[39] KUANG S Y,XIAO W W,FENG L,et al.Effects of graded levels of dietary methionine hydroxy analogue on immune response and antioxidant status of immune organs in juvenile Jian carp (Cyprinus carpio var.Jian)[J].Fish &Shellfish Immunology,2012,32(5):629-636.