近年来,中国水产养殖行业发展迅速,其中贝类养殖产量占到全国产量的28.54%,已成为中国经济水产品的重要组成部分[1]。大多数贝类生活在潮间带环境中,其生境复杂性极易受到人类活动和自然环境的双重影响[2],如温度、盐度和溶氧等环境因素的变化。在众多环境因素中,温度变化是影响最大的因素之一。
温度是影响水生动物正常活动的一个关键因子,主要影响生物体内的生物化学反应进程和生物大分子的活性[3]。大部分水产动物都是变温动物,而环境温度是影响变温动物生理状态的主要因素,对于低等无脊椎动物而言,特别是贝类,温度可影响其心跳、呼吸、氧的结合与运输、摄食、消化和排泄器官功能等[4]。高温胁迫会对贝类的生长、新陈代谢及免疫活性产生一定影响,同样,低温胁迫会引起机体氧化应激,造成活性氧的大量生成和过度累积,进而引起细胞损伤、突变和死亡,最终降低机体的免疫力[5]。
越来越多的研究表明了水产动物对冷应激的适应反应,并表明水生动物可以通过广泛的生物化学、代谢和生理适应过程逐渐形成其冷适应表型,这些过程包括产生温度特异性同工酶、维持膜流动性和合成分子伴侣等[4]。大黄鱼体内脂肪酸去饱和酶(fatty acid synthase,FADs)通过调控生物膜上的不饱和脂肪酸含量,维持生物膜的流动性,进而对低温环境做出反应与适应性[6]。中华绒螯蟹热休克蛋白70(heat shock protein 70,HSP70)和热休克蛋白90(heat shock protein 90,HSP90)基因在低温胁迫下被诱导,通过参与促进错误折叠蛋白的降解过程来维持细胞稳态,从而减少低温对中华绒螯蟹造成的损伤[7]。此外,中国圆田螺在低温保活过程中会改变体内脂肪酸、蛋白质、糖原、脂肪和水分等物质含量,从而提高其抗寒能力[8]。近年来,在中国北方,养殖菲律宾蛤仔(Ruditapes philippinarum)在冬季经常大量死亡,可能是对该季节较低温度的反应。然而,相关研究中对菲律宾蛤仔低温暴露,特别是对长期低温暴露的生理适应背后的生理变化和分子途径知之甚少,因此,研究菲律宾蛤仔对长期低温暴露适应的分子机制至关重要。
本研究中以菲律宾蛤仔为研究对象,进行为期60 d的低温暴露,对其免疫指标和差异表达基因进行了系统测定和分析,旨在探究菲律宾蛤仔响应低温暴露的生理学特征和分子机制,以期为菲律宾蛤仔耐低温机理研究提供基础数据,并进一步为贝类耐低温品种的选育提供有益参考。
1 材料与方法
1.1 材料
本试验所用菲律宾蛤仔(壳长为3.83 cm±0.14 cm)购自连云港市赣榆区苏鲁海产品综合批发市场。
1.2 方法
1.2.1 试验贝暴露分组与样品采集 在正式试验前,将每组30粒蛤仔置于装有10 L人工海水的塑料箱中暂养7 d(温度为17.54 ℃±0.24 ℃,盐度为25±1,pH为7.5,DO>7.5 mg/L)。暂养结束后,进行低温暴露试验。试验共设置2个组别,分别为对照组18.03 ℃±0.56 ℃(记为“GCON”)和低温组2.03 ℃±0.50 ℃(记为“GLT”),每组分别设置3个重复,每个重复放置30粒蛤仔,除温度外,其余试验条件与暂养条件相同,其中,低温组塑料箱置于冰柜中,对照组塑料箱置于室温环境中,通过空调控温保持温度稳定。在暴露20、60 d后,分别在每个试验组重复取3粒蛤仔的鳃组织混合用于转录组中RNA提取。此外,取试验处理20、40、60 d的蛤仔鳃组织样品于-80 ℃冰箱中保存,这些样品用于后续免疫指标测定和实时荧光定量PCR(qRT-PCR)验证。
1.2.2 免疫指标测定 采用南京建成生物工程研究所研发的试剂盒测定菲律宾蛤仔鳃组织中过氧化氢酶(CAT)活力、超氧化物歧化酶(SOD)活力、丙二醛(MDA)含量和总抗氧化能力(T-AOC)水平。
1.2.3 转录组测序
1)总RNA提取、建库与测序。根据Trizol试剂提取每管鳃组织的总RNA,并使用eppendorf超微量紫外分光光度计进行RNA的纯度和浓度检测。将获得的mRNA随机打断,将其作为模板,随机寡核苷酸作为引物,cDNA的第一条链在1st Strand Enzyme逆转录酶体系中合成,第二条链利用2st Strand Enzyme和dNTPs合成。纯化后的双链cDNA进行PCR扩增并再次纯化PCR产物,最终获得文库。文库构建完成后,使用Agilent 2100/4150对文库的insert size进行检测,以保证文库质量。库检合格后,送深圳承启生物科技有限公司测序。
2)转录组分析。本次分析中差异基因的筛选阈值设定为错误发现率(false discovery rate,FDR)<0.05且|log2(FoldChange)|>1。根据注释结果,分别对差异表达基因(differentially expressed genes,DEGs)的功能和生物学途径进行分类,从而获得GO功能注释和KEGG通路富集分析。
3)DEGs qRT-PCR验证。在DEGs中随机选取10个基因,将18S作为内参基因进行qRT-PCR检验,利用primer 5软件设计特异性引物(表1)。使用LightCycler® 96进行qRT-PCR,反应体系按TB Green® Premix Ex TaqTM Ⅱ(TaKaRa,中国)试剂盒说明书进行。反应体系为2×TB Green Premix Ex Taq 10 μL;Forward primer 0.8 μL;Reverse primer 0.8 μL;Template cDNA 1 μL;ddH2O 7.4 μL,总体积20 μL。采用两步法PCR扩增标准程序,反应程序:95 ℃下预变性30 s;95 ℃下变性5 s,60 ℃下退火延伸20 s,共进行40个循环。用2-ΔΔCt法计算基因的相对表达量。
表1 实时荧光定量PCR引物
Tab.1 Real-time fluorescent quantitative PCR primers
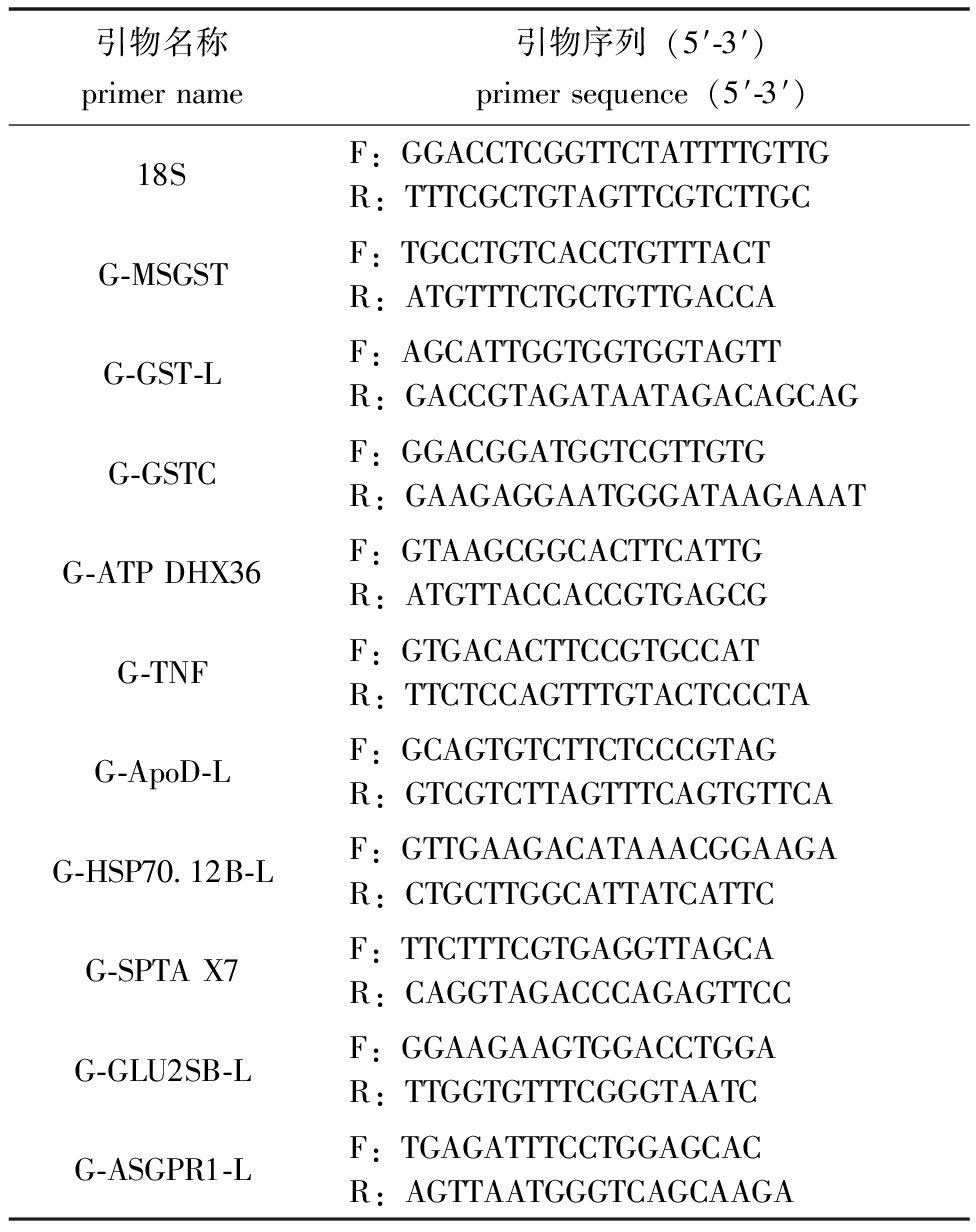
引物名称primername引物序列(5′-3′)primersequence(5′-3′)18SF:GGACCTCGGTTCTATTTTGTTGR:TTTCGCTGTAGTTCGTCTTGCG-MSGSTF:TGCCTGTCACCTGTTTACTR:ATGTTTCTGCTGTTGACCAG-GST-LF:AGCATTGGTGGTGGTAGTTR:GACCGTAGATAATAGACAGCAGG-GSTCF:GGACGGATGGTCGTTGTGR:GAAGAGGAATGGGATAAGAAATG-ATPDHX36F:GTAAGCGGCACTTCATTGR:ATGTTACCACCGTGAGCGG-TNFF:GTGACACTTCCGTGCCATR:TTCTCCAGTTTGTACTCCCTAG-ApoD-LF:GCAGTGTCTTCTCCCGTAGR:GTCGTCTTAGTTTCAGTGTTCAG-HSP70.12B-LF:GTTGAAGACATAAACGGAAGAR:CTGCTTGGCATTATCATTCG-SPTAX7F:TTCTTTCGTGAGGTTAGCAR:CAGGTAGACCCAGAGTTCCG-GLU2SB-LF:GGAAGAAGTGGACCTGGAR:TTGGTGTTTCGGGTAATCG-ASGPR1-LF:TGAGATTTCCTGGAGCACR:AGTTAATGGGTCAGCAAGA
1.3 数据处理
试验数据以平均值±标准差(mean±S.D.)表示,采用SPSS 26.0软件进行单因素方差分析(ANOVA),采用T test法进行两组间的比较,显著性水平设为0.05。
2 结果与分析
2.1 长期低温暴露对菲律宾蛤仔免疫指标影响
2.1.1 长期低温暴露对菲律宾蛤仔SOD活力的影响 低温暴露20 d时,菲律宾蛤仔SOD活力和对照组分别为17.33、19.71 U/mgprot,菲律宾蛤仔SOD活力与对照组无显著性差异(P>0.05)。低温暴露40 d时,蛤仔SOD活力和对照组分别为25.87、16.60 U/mgprot,蛤仔SOD活力显著高于对照组(P<0.05)。低温暴露60 d时,蛤仔SOD活力和对照组分别为21.15、15.13 U/mgprot,低温组SOD活力显著高于对照组(P<0.05)(图1)。
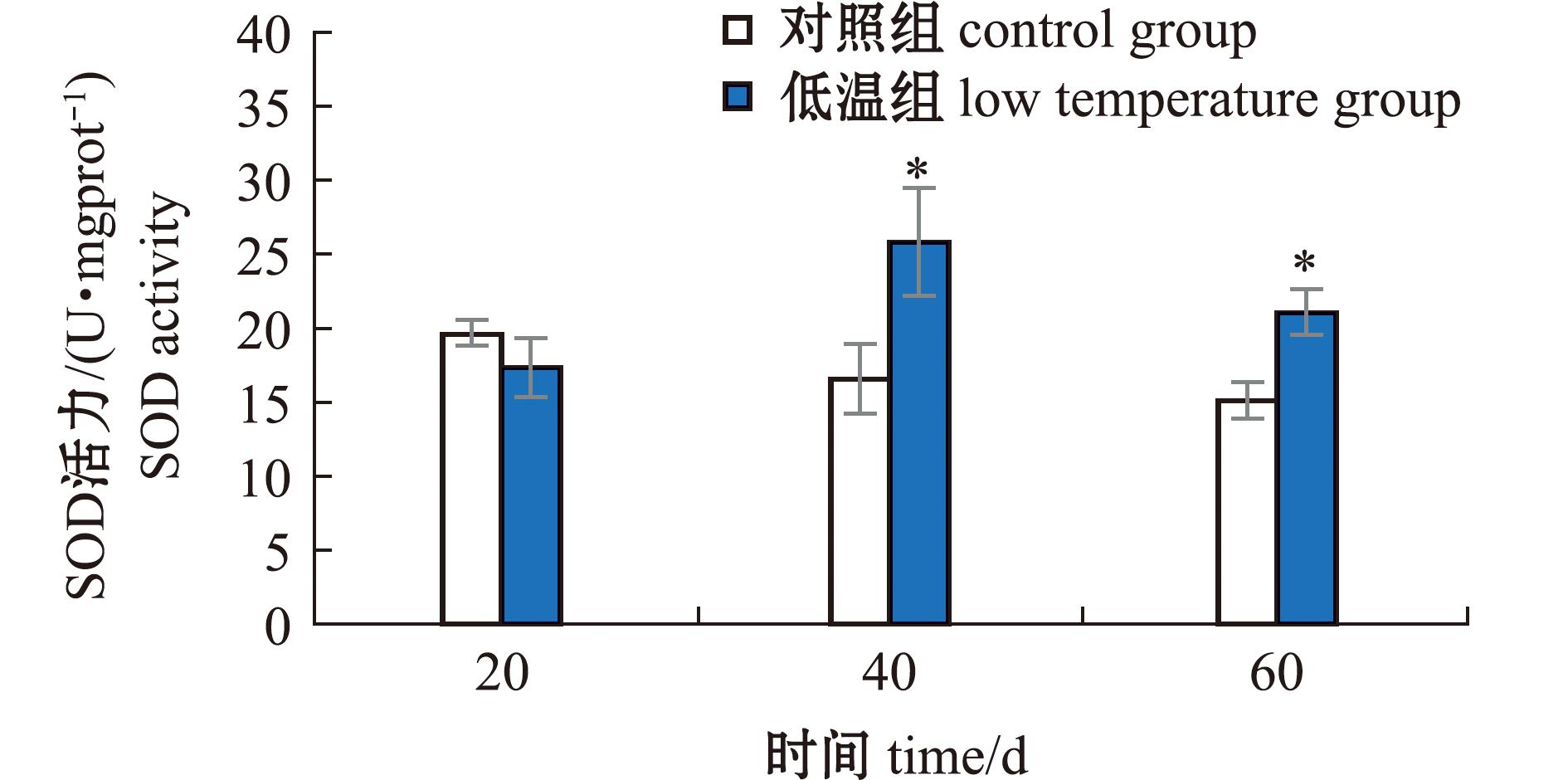
低温组上的*代表与该取样点的空白对照组有显著性差异(P<0.05),下同。
*above the low-temperature group represents a significant difference from the blank control group at that sampling point(P<0.05),et sequentia.
图1 长期低温暴露对菲律宾蛤仔SOD活力的影响
Fig.1 Effect of prolonged cold exposure on SOD activity of Ruditapes philippinarum
2.1.2 长期低温暴露对菲律宾蛤仔CAT活力的影响 低温暴露60 d过程中,菲律宾蛤仔CAT活力在20、40、60 d时分别为2.93、2.28、1.15 U/mgprot,对照组分别为5.44、4.96、4.91 U/mgprot,菲律宾蛤仔CAT活力均显著低于对照组(P<0.05)(图2)。
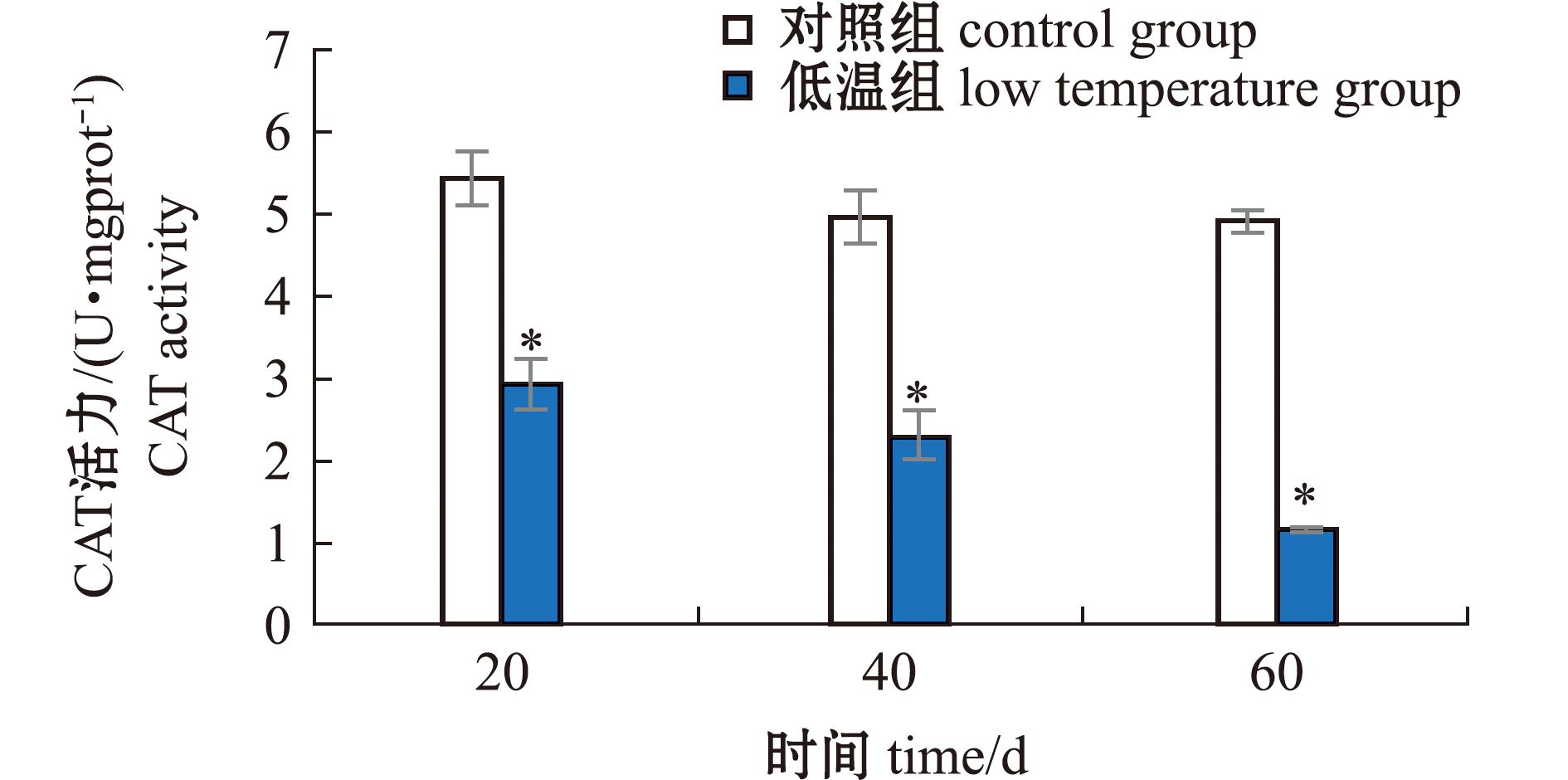
图2 长期低温暴露对菲律宾蛤仔CAT活力的影响
Fig.2 Effect of prolonged cold exposure on CAT activity of Ruditapes philippinarum
2.1.3 长期低温暴露对菲律宾蛤仔MDA含量的影响 低温暴露20 d和40 d时,菲律宾蛤仔MDA含量高于对照组(P>0.05)。低温暴露60 d时,蛤仔MDA含量和对照组分别为3.75、4.23 nmol/mgprot,低温组MDA含量显著低于对照组(P<0.05),且60 d低温组MDA含量最低(图3)。
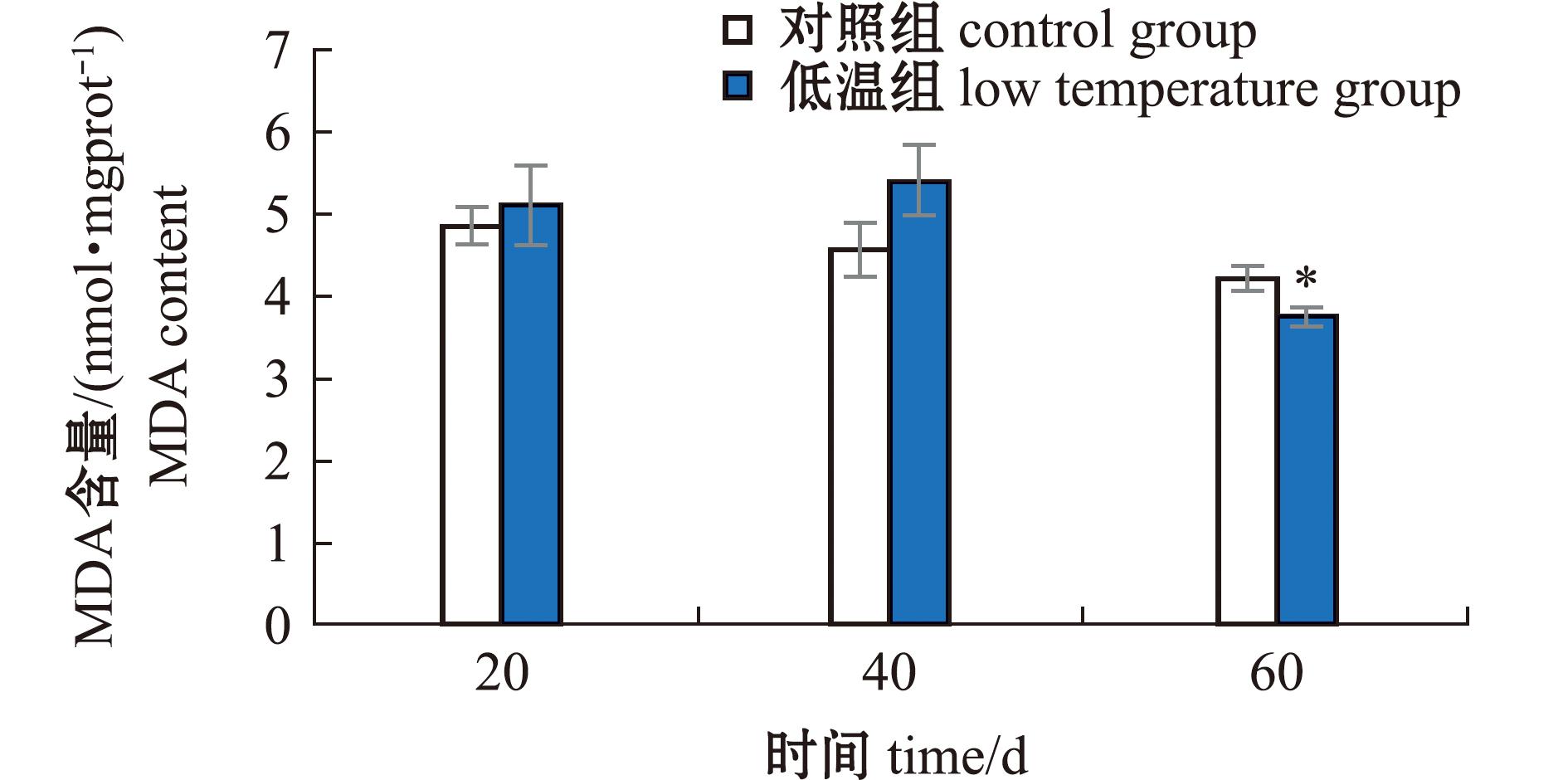
图3 长期低温暴露对菲律宾蛤仔MDA含量的影响
Fig.3 Effect of prolonged cold exposure on MDA content of Ruditapes philippinarum
2.1.4 长期低温暴露对菲律宾蛤仔T-AOC水平的影响 低温暴露60 d过程中,菲律宾蛤仔T-AOC水平在20、40、60 d分别为0.30、0.25、0.16 U/mgprot,对照组分别为1.38、1.11、1.30 U/mgprot,低温组蛤仔T-AOC水平均显著低于对照组(P<0.05)(图4)。
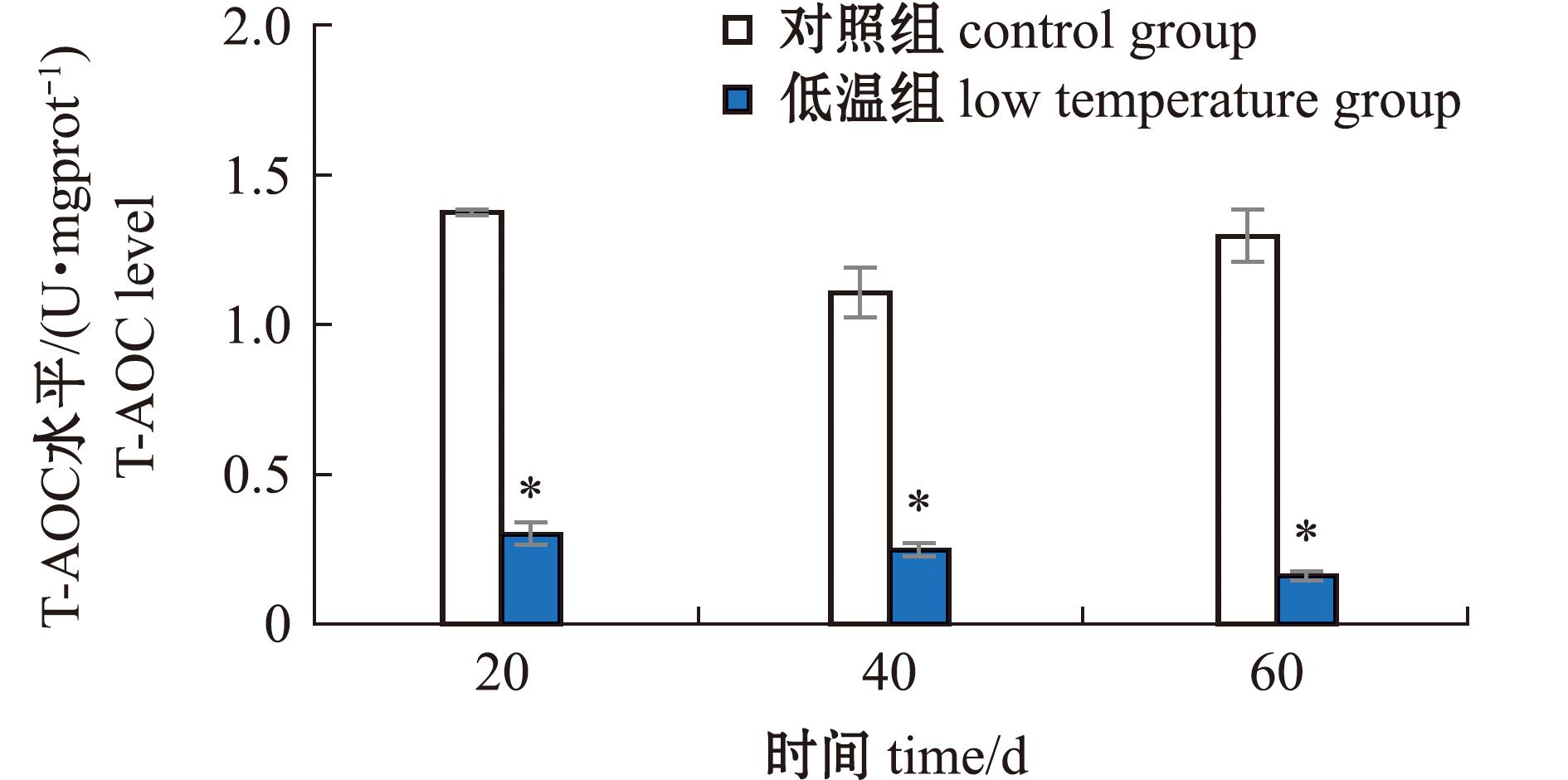
图4 长期低温暴露对菲律宾蛤仔T-AOC水平的影响
Fig.4 Effect of prolonged cold exposure on T-AOC level of Ruditapes philippinarum
2.2 长期低温暴露对菲律宾蛤仔的转录响应
2.2.1 转录组测序质量及注释分析 样品测序情况汇总见表2,从表2可见,原始序列(raw reads)为23 352 190~39 144 664条,质控序列(clean reads)为23 311 263~38 594 947条,质控率为98.34%~99.87%,菲律宾蛤仔序列中Q20值为97.87%~98.63%,Q30值为93.94%~95.71%,GC含量为34.60%~35.83%,表明测序数据质量可用于对后续数据分析。
表2 测序结果汇总
Tab.2 Summary of sequencing result %
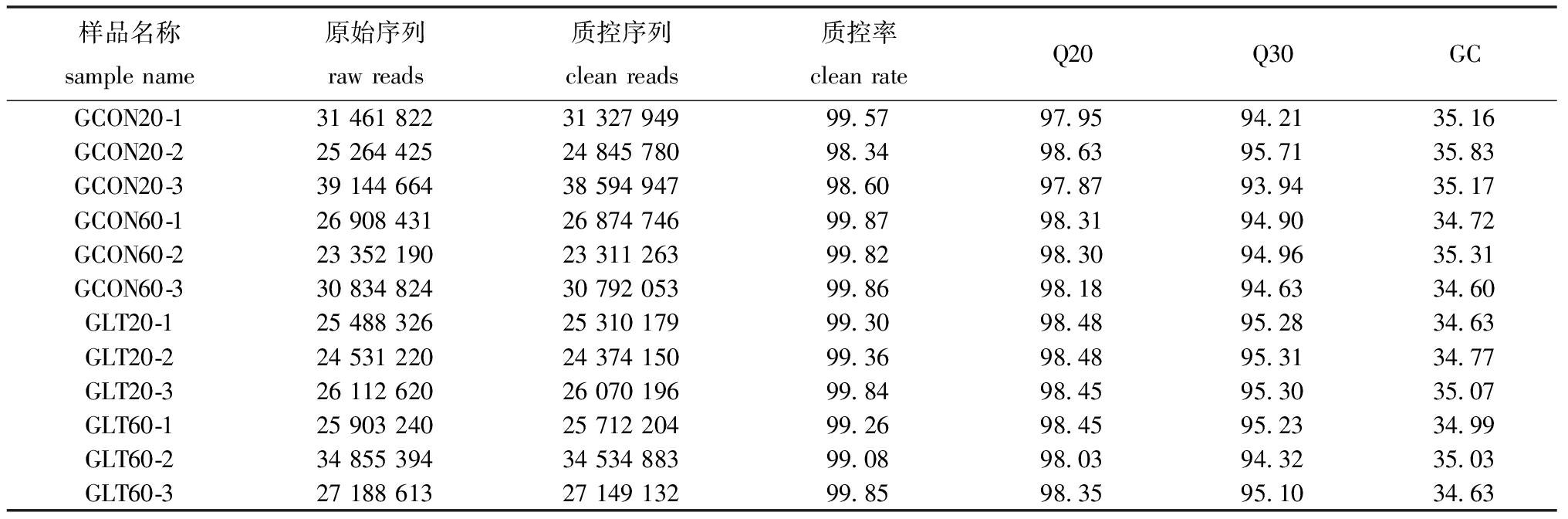
样品名称samplename原始序列rawreads质控序列cleanreads质控率cleanrateQ20Q30GCGCON20-1314618223132794999.5797.9594.2135.16GCON20-2252644252484578098.3498.6395.7135.83GCON20-3391446643859494798.6097.8793.9435.17GCON60-1269084312687474699.8798.3194.9034.72GCON60-2233521902331126399.8298.3094.9635.31GCON60-3308348243079205399.8698.1894.6334.60GLT20-1254883262531017999.3098.4895.2834.63GLT20-2245312202437415099.3698.4895.3134.77GLT20-3261126202607019699.8498.4595.3035.07GLT60-1259032402571220499.2698.4595.2334.99GLT60-2348553943453488399.0898.0394.3235.03GLT60-3271886132714913299.8598.3595.1034.63
注:GCON—对照组鳃;GLT—低温组鳃,下同。
Note:GCON indicates gills of the control group,GLT indicates gills of the low-temperature group,et sequentia.
2.2.2 DEGs 筛选 差异基因的筛选阈值设定为FDR<0.05且|log2(FoldChange)|>1,不同比较组结果见表3。
表3 差异基因数量统计
Tab.3 Statistic of the number of differential gene
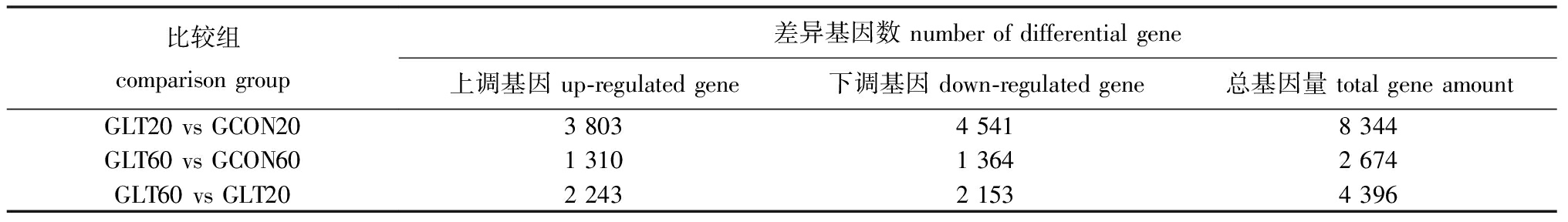
比较组comparisongroup差异基因数numberofdifferentialgene上调基因up-regulatedgene下调基因down-regulatedgene总基因量totalgeneamountGLT20vsGCON20380345418344GLT60vsGCON60131013642674GLT60vsGLT20224321534396
2.2.3 DEGs的GO富集分析 本试验中挑选了富集最显著的20个GO term在图5中展示,如果不足20条,则全部展示。
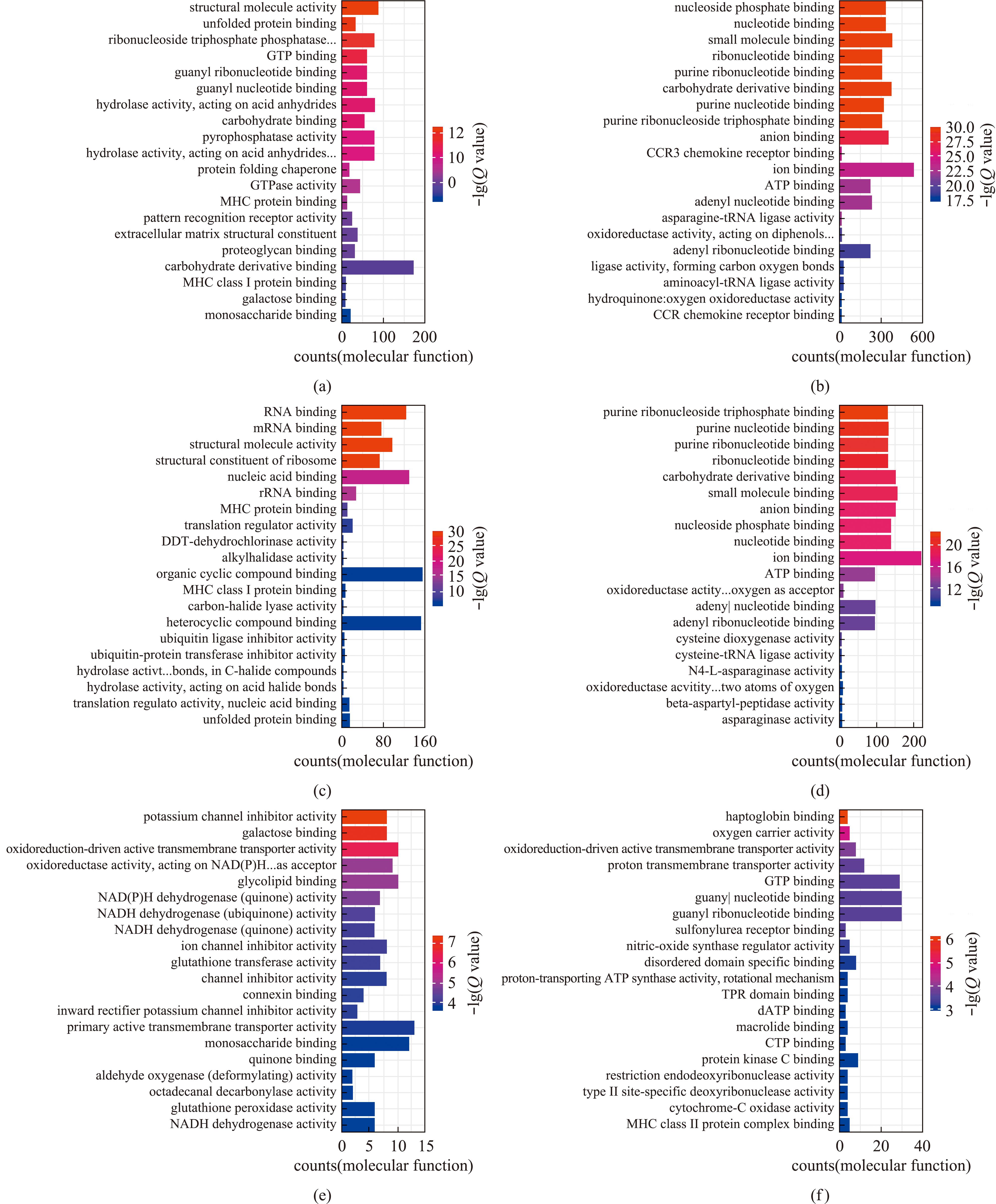
(a)和(b)分别为比较组GLT20 vs GCON20的上调基因和下调基因在分子功能中富集结果;(c)和(d)分别为比较组GLT60 vs GCON60的上调基因和下调基因在分子功能中富集结果;(e)和(f)分别为比较组GLT60 vs GLT20的上调基因和下调基因在分子功能中富集结果。
(a) and (b) are the results of enrichment of up-regulated gene and down-regulated gene in molecular functions,respectively,for the comparison group GLT20 vs GCON20;(c) and (d) are the results of enrichment of up-regulated gene and down-regulated gene in molecular functions,respectively,for the comparison group GLT60 vs GCON60;(e) and (f) are the results of enrichment of up-regulated genes and down-regulated genes in molecular functions,respectively,for the comparison group GLT60 vs GLT20.
图5 长期低温暴露下菲律宾蛤仔差异基因的GO富集
Fig.5 GO enrichment of differential genes in Ruditapes philippinarum experienced prolonged cold exposure
对比较组GLT20 vs GCON20、GLT60 vs GCON60和GLT60 vs GLT20的上调表达基因和下调表达基因分别进行GO富集分析。结果表明,差异基因GO功能主要富集在生物过程(biological process,BP)、细胞组分(cellular component,CC)和分子功能(molecular function,MF)3大类别。下面主要分析分子功能类别。
试验过程中,从图5(a)可见:分子功能中,比较组GLT20 vs GCON20的上调基因富集(Q value<0.05)在结构分子活性(structural molecule activity)等条目。从图5(b)可见:分子功能中,比较组GLT20 vs GCON20的下调基因富集(Q value<0.05)在嘌呤核糖核苷三磷酸结合(purine ribonucleoside triphosphate binding)等条目。从图5(c)可见:分子功能中,比较组GLT60 vs GCON60的上调基因富集(Q value<0.05)在核糖体的结构成分(structural constituent of ribosome)等条目。从图5(d)可见:分子功能中,比较组GLT60 vs GCON60的下调基因富集(Q value<0.05)在嘌呤核糖核苷三磷酸结合(purine ribonucleoside triphosphate binding)等条目。从图5(e)可见:分子功能中,比较组GLT60 vs GLT20的上调基因富集在钾通道抑制剂活性(potassium channel inhibitor activity)等条目。从图5(f)可见:分子功能中,比较组GLT60 vs GLT20的下调基因富集(Q value<0.05)在结合珠蛋白结合(haptoglobin binding)等条目。
2.2.4 DEGs 的 KEGG 富集分析 本试验挑选了Q value最小的20条pathway条目在图6中进行展示,若富集的pathway条目不足20条,则全部展示。对比较组GLT20 vs GCON20的上调表达基因和下调表达基因分别进行KEGG通路分析。KEGG通路中,上调基因富集(Q value<0.05)在α-亚麻酸代谢(alpha-Linolenic acid metabolism)等通路(图6(a))。而在KEGG通路中,下调基因富集(Q value<0.05)在牛磺酸和次牛磺酸代谢(taurine and hypotaurine metabolism)等通路(图6(b))。
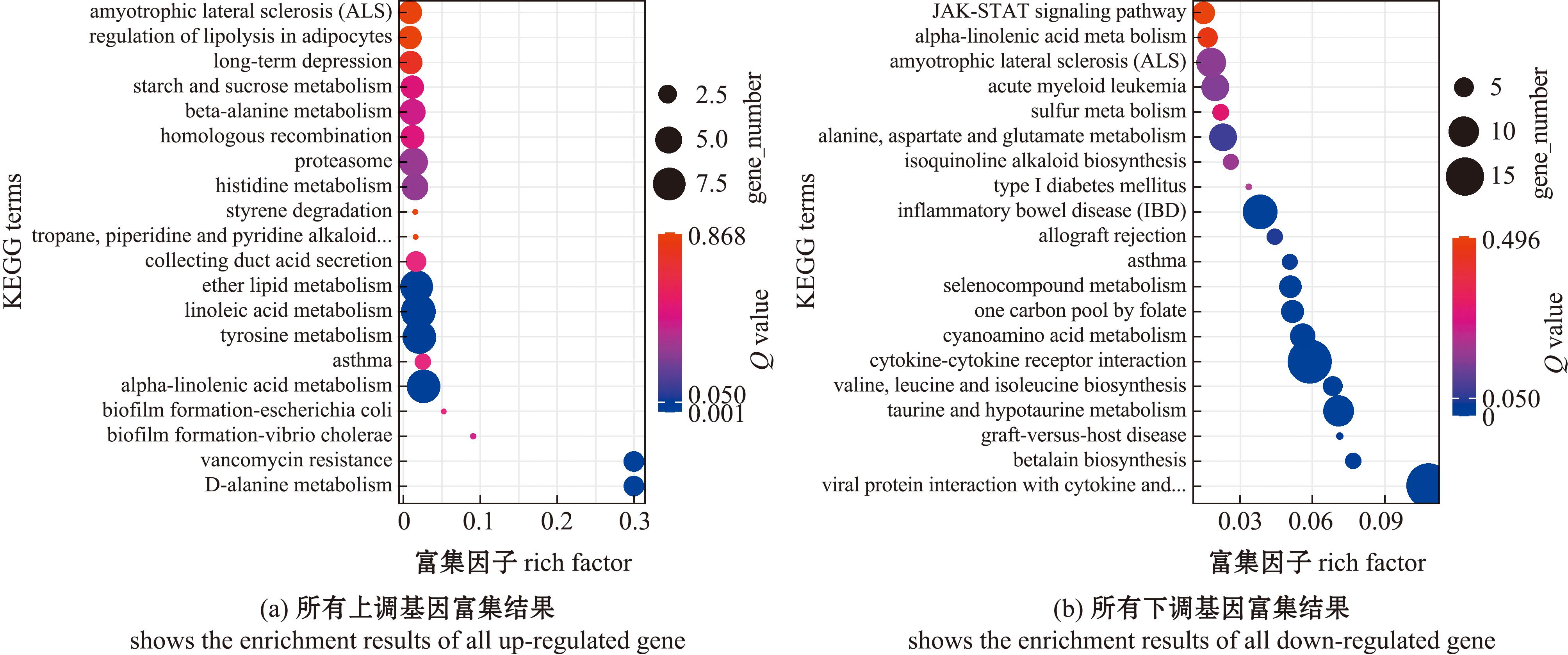
图6 长期低温暴露下菲律宾蛤仔差异基因的KEGG富集(GLT20 vs GCON20)
Fig.6 KEGG enrichment of differential genes in Ruditapes philippinarum under prolonged cold exposure(GLT20 vs GCON20)
对比较组GLT60 vs GCON60的上调表达基因和下调表达基因分别进行KEGG通路分析。KEGG通路中,无上调基因的显著富集(Q value>0.05)(图7(a))。KEGG通路中,下调基因富集(Q value<0.05)在牛磺酸和次牛磺酸代谢(taurine and hypotaurine metabolism)等通路(图7(b))。
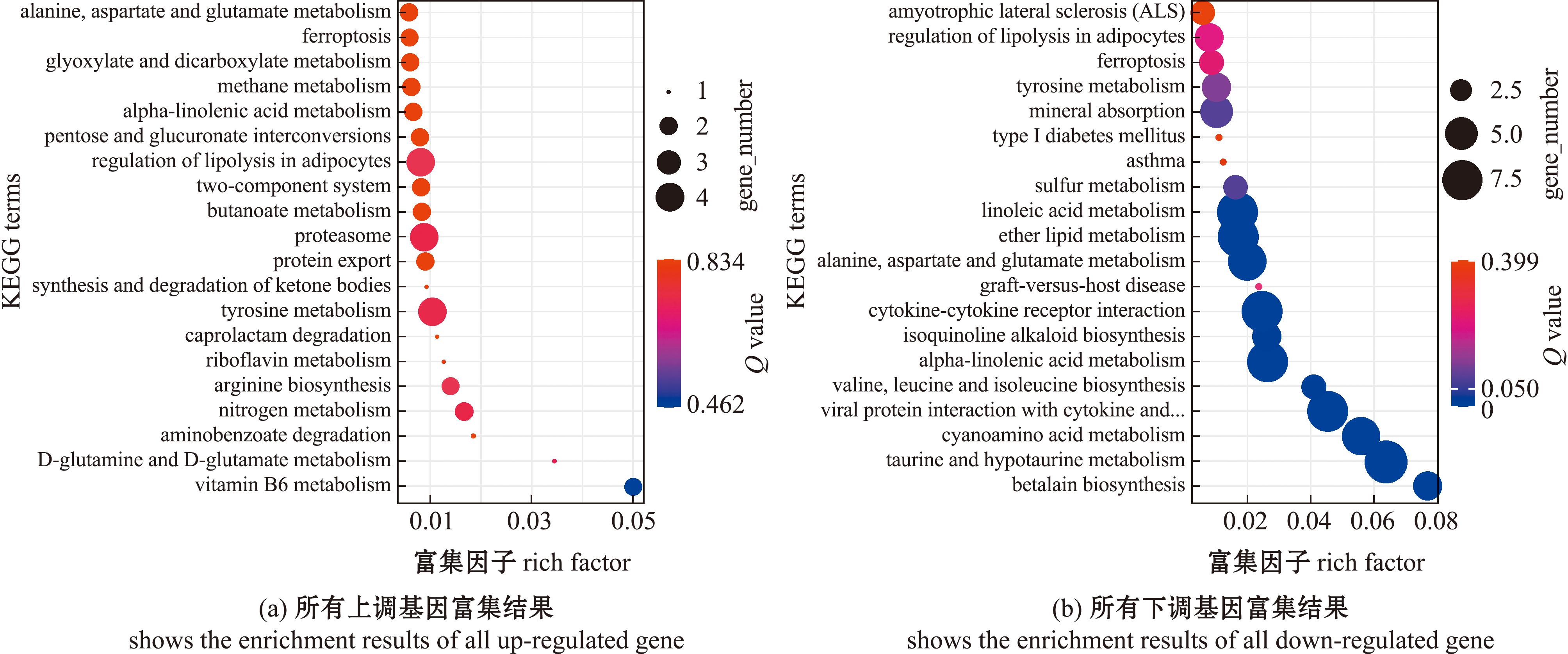
图7 长期低温暴露下菲律宾蛤仔差异基因的KEGG富集(GLT60 vs GCON60)
Fig.7 KEGG enrichment of differential gene in Ruditapes philippinarum experienced prolonged cold exposure(GLT60 vs GCON60)
对比较组GLT60 vs GLT20的上调表达基因和下调表达基因分别进行KEGG通路富集分析。KEGG通路中,上调基因富集(Q value<0.05)在ether lipid代谢(ether lipid metabolism)等通路(图8(a))。KEGG通路中,下调基因的富集(Q value<0.05)分别是碱基切除修复(base excision repair)等通路(图8(b))。
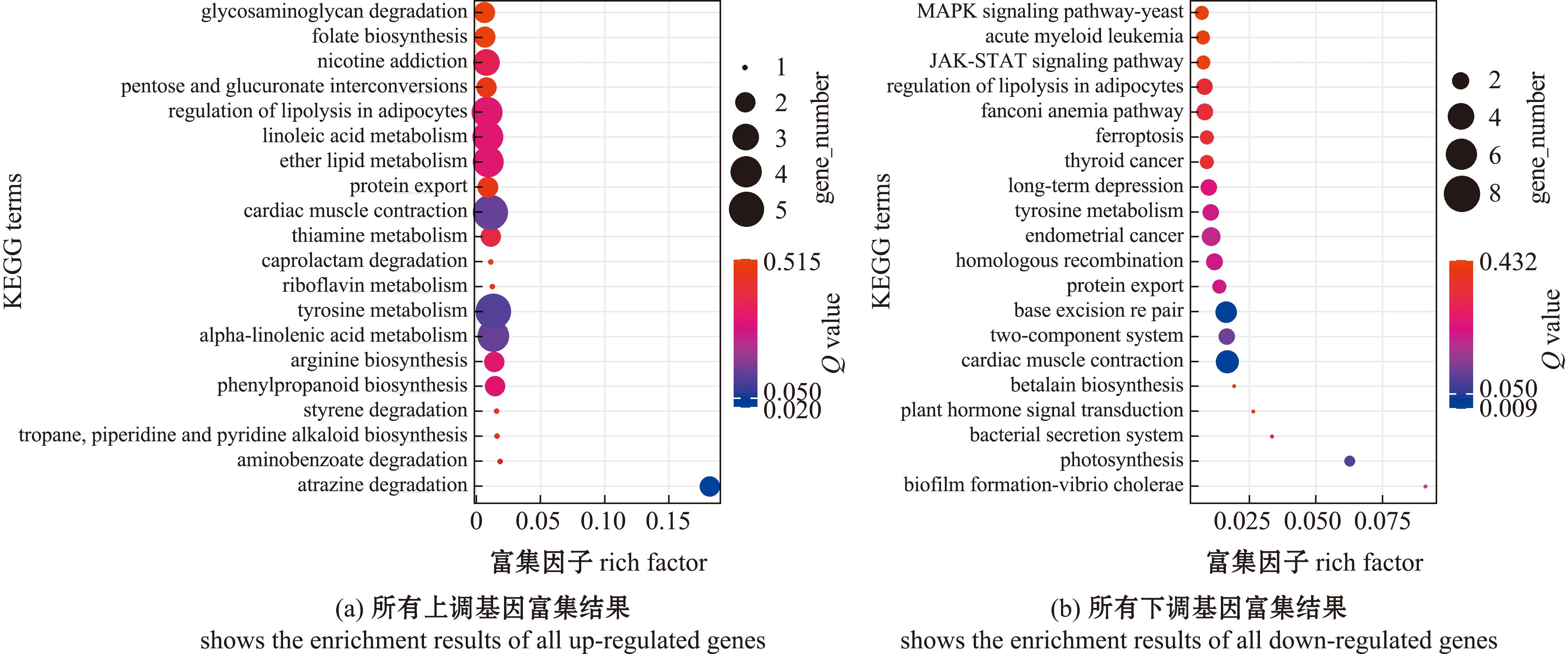
图8 长期低温暴露下菲律宾蛤仔差异基因的KEGG富集(GLT60 vs GLT20)
Fig.8 KEGG enrichment of differential gene in Ruditapes philippinarum experienced prolonged cold exposure(GLT60 vs GLT20)
进一步对数据分析发现,许多DEGs的产物参与菲律宾蛤仔蛋白质加工过程,如硫氧还蛋白(TRX)等(表4)。还发现多种DEGs参与蛤仔物质代谢过程,如磷脂酶A和酰基转移酶3(PLAAT-3)和酰基辅酶A去饱和酶(ACD)。此外,本研究中还检测到一些参与抗氧化、免疫和抗凋亡的DEGs,如谷胱甘肽S转移酶基因(GST)等(表4)。
表4 长期低温暴露下菲律宾蛤仔蛋白质加工、物质代谢、抗氧化、免疫和抗凋亡相关DEGs列表
Tab.4 List of DEGs associated with protein processing,substance metabolism,antioxidation,immunology and anti-apoptotic in Ruditapes philippinarum experienced prolonged cold exposure
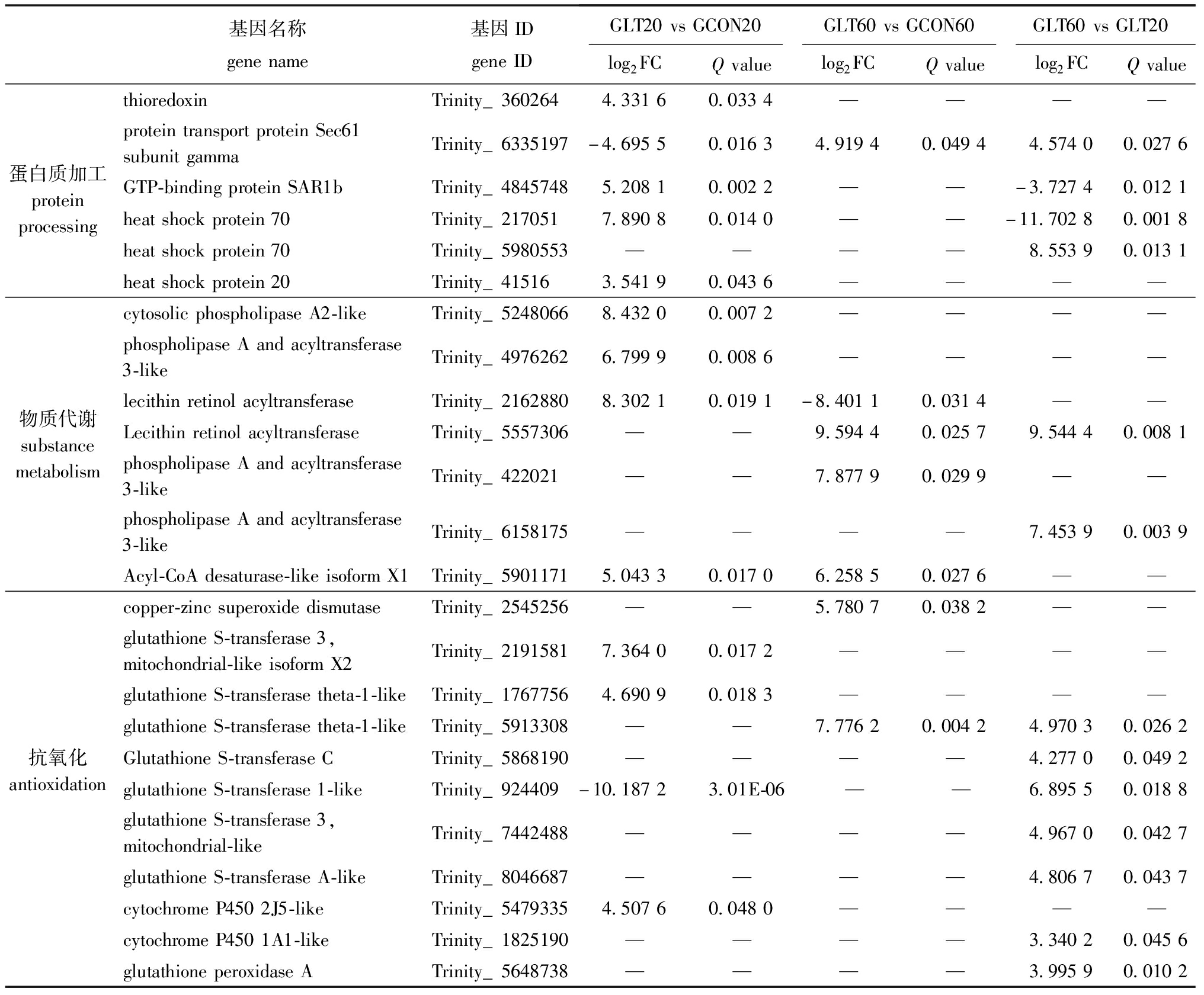
基因名称genename基因IDgeneIDGLT20vsGCON20GLT60vsGCON60GLT60vsGLT20log2FCQvaluelog2FCQvaluelog2FCQvaluethioredoxinTrinity_3602644.33160.0334————proteintransportproteinSec61subunitgammaTrinity_6335197-4.69550.01634.91940.04944.57400.0276蛋白质加工proteinprocessingGTP-bindingproteinSAR1bTrinity_48457485.20810.0022——-3.72740.0121heatshockprotein70Trinity_2170517.89080.0140——-11.70280.0018heatshockprotein70Trinity_5980553————8.55390.0131heatshockprotein20Trinity_415163.54190.0436————cytosolicphospholipaseA2-likeTrinity_52480668.43200.0072————phospholipaseAandacyltransferase3-likeTrinity_49762626.79990.0086————lecithinretinolacyltransferaseTrinity_21628808.30210.0191-8.40110.0314——物质代谢substancemetabolismLecithinretinolacyltransferaseTrinity_5557306——9.59440.02579.54440.0081phospholipaseAandacyltransferase3-likeTrinity_422021——7.87790.0299——phospholipaseAandacyltransferase3-likeTrinity_6158175————7.45390.0039Acyl-CoAdesaturase-likeisoformX1Trinity_59011715.04330.01706.25850.0276——copper-zincsuperoxidedismutaseTrinity_2545256——5.78070.0382——glutathioneS-transferase3,mitochondrial-likeisoformX2Trinity_21915817.36400.0172————glutathioneS-transferasetheta-1-likeTrinity_17677564.69090.0183————glutathioneS-transferasetheta-1-likeTrinity_5913308——7.77620.00424.97030.0262抗氧化antioxidationGlutathioneS-transferaseCTrinity_5868190————4.27700.0492glutathioneS-transferase1-likeTrinity_924409-10.18723.01E-06——6.89550.0188glutathioneS-transferase3,mitochondrial-likeTrinity_7442488————4.96700.0427glutathioneS-transferaseA-likeTrinity_8046687————4.80670.0437cytochromeP4502J5-likeTrinity_54793354.50760.0480————cytochromeP4501A1-likeTrinity_1825190————3.34020.0456glutathioneperoxidaseATrinity_5648738————3.99590.0102
续表4 长期低温暴露下菲律宾蛤仔蛋白质加工、物质代谢、抗氧化、免疫和抗凋亡相关DEGs列表
Cont.Tab.4 List of DEGs associated with protein processing,substance metabolism,antioxidation,immunology and anti-apoptotic in Ruditapes philippinarum under prolonged cold exposure
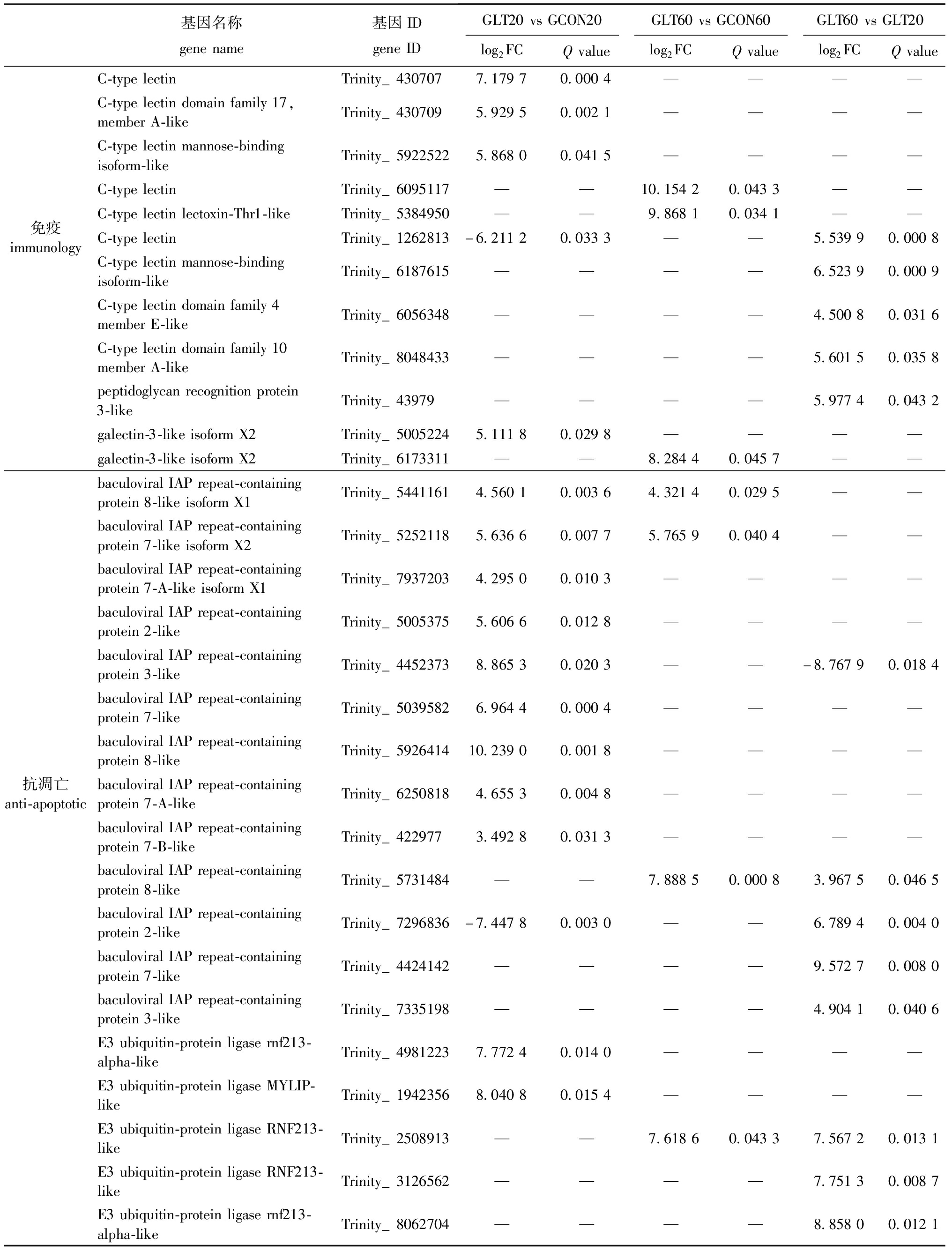
基因名称genename基因IDgeneIDGLT20vsGCON20GLT60vsGCON60GLT60vsGLT20log2FCQvaluelog2FCQvaluelog2FCQvalueC-typelectinTrinity_4307077.17970.0004————C-typelectindomainfamily17,memberA-likeTrinity_4307095.92950.0021————C-typelectinmannose-bindingisoform-likeTrinity_59225225.86800.0415————C-typelectinTrinity_6095117——10.15420.0433——C-typelectinlectoxin-Thr1-likeTrinity_5384950——9.86810.0341——免疫immunologyC-typelectinTrinity_1262813-6.21120.0333——5.53990.0008C-typelectinmannose-bindingisoform-likeTrinity_6187615————6.52390.0009C-typelectindomainfamily4memberE-likeTrinity_6056348————4.50080.0316C-typelectindomainfamily10memberA-likeTrinity_8048433————5.60150.0358peptidoglycanrecognitionprotein3-likeTrinity_43979————5.97740.0432galectin-3-likeisoformX2Trinity_50052245.11180.0298————galectin-3-likeisoformX2Trinity_6173311——8.28440.0457——baculoviralIAPrepeat-containingprotein8-likeisoformX1Trinity_54411614.56010.00364.32140.0295——baculoviralIAPrepeat-containingprotein7-likeisoformX2Trinity_52521185.63660.00775.76590.0404——baculoviralIAPrepeat-containingprotein7-A-likeisoformX1Trinity_79372034.29500.0103————baculoviralIAPrepeat-containingprotein2-likeTrinity_50053755.60660.0128————baculoviralIAPrepeat-containingprotein3-likeTrinity_44523738.86530.0203——-8.76790.0184baculoviralIAPrepeat-containingprotein7-likeTrinity_50395826.96440.0004————baculoviralIAPrepeat-containingprotein8-likeTrinity_592641410.23900.0018————抗凋亡anti-apoptoticbaculoviralIAPrepeat-containingprotein7-A-likeTrinity_62508184.65530.0048————baculoviralIAPrepeat-containingprotein7-B-likeTrinity_4229773.49280.0313————baculoviralIAPrepeat-containingprotein8-likeTrinity_5731484——7.88850.00083.96750.0465baculoviralIAPrepeat-containingprotein2-likeTrinity_7296836-7.44780.0030——6.78940.0040baculoviralIAPrepeat-containingprotein7-likeTrinity_4424142————9.57270.0080baculoviralIAPrepeat-containingprotein3-likeTrinity_7335198————4.90410.0406E3ubiquitin-proteinligasernf213-alpha-likeTrinity_49812237.77240.0140————E3ubiquitin-proteinligaseMYLIP-likeTrinity_19423568.04080.0154————E3ubiquitin-proteinligaseRNF213-likeTrinity_2508913——7.61860.04337.56720.0131E3ubiquitin-proteinligaseRNF213-likeTrinity_3126562————7.75130.0087E3ubiquitin-proteinligasernf213-alpha-likeTrinity_8062704————8.85800.0121
注:—表示该基因在处理组中无显著性差异。
Note:—means that the gene is not significantly different in the treatment group.
2.2.5 qRT-PCR 验证 对转录组结果进行qRT-PCR验证,结果如图9所示。验证结果中基因的表达趋势和转录组中DEGs的表达趋势基本相同,证明了转录组结果的可靠性。
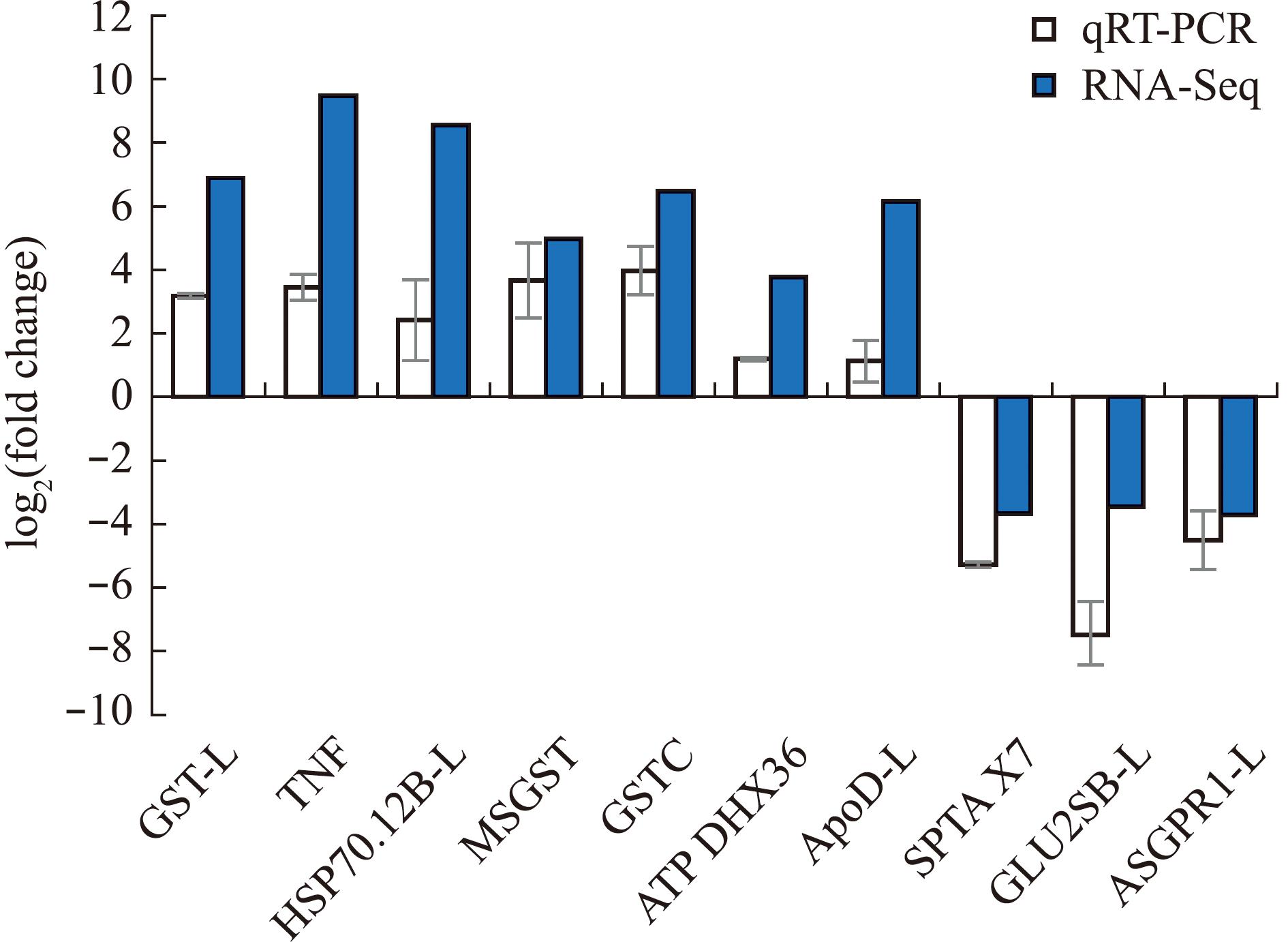
图9 转录组结果验证(GLT60 vs GLT20)
Fig.9 Validation of transcriptome results(GLT60 vs GLT20)
3 讨论
本试验中通过转录组学技术研究了菲律宾蛤仔适应低温潜在机制。转录组数据发现,比较组GLT20 vs GCON20、比较组GLT60 vs GCON60和比较组GLT60 vs GLT20分别有8 344、2 674和4 396个DEGs,这些DEGs主要参与蛋白质加工、物质代谢、抗氧化、免疫和抗凋亡等生物过程。
3.1 蛋白质加工
在真核细胞中,内质网(endoplasmic reticulum,ER)在多种重要的分泌蛋白和膜蛋白的合成中起着至关重要作用[9]。内质网内合成的蛋白质通过外壳蛋白复合物Ⅱ(coat protein complex Ⅱ,COPⅡ)包被的囊泡转运至高尔基体,所述囊泡由GTP结合蛋白SAR1b(GTP-binding protein SAR1b,SAR1B)、内壳复合物(SEC23/SEC24)和外壳复合物(SEC13/SEC31)等构成。此外,SAR1B可能是海胆对高温反应的潜在标志物[10]。斑马鱼肠细胞吸收食物中的胆固醇需要SAR1B,SAR1B促进外分泌胰腺和肝脏的生长[11]。已有研究表明,菲律宾蛤仔中SAR1B基因在热胁迫后显著上调[12]。本研究中,低温暴露20 d后,低温组SAR1B基因表达显著上调,说明低温也能诱导SAR1B基因的高表达,提示菲律宾蛤仔可能通过调节内质网中的蛋白质转运和加工来适应低温暴露。
当应激时,蛋白质在内质网发生错误折叠,堆积于细胞内,从而影响细胞正常的功能,甚至引起疾病[13]。内质网应激相关性降解(endoplasmic reticulum-associated protein degradation,ERAD)是真核细胞调节内质网压力的重要途径之一。已有研究发现,内质网中的多个蛋白质复合体可识别、清除、泛素化错误折叠的蛋白质,可将其转运到26S蛋白酶体并在细胞质中降解[14]。在这一过程中,热激蛋白(HSP)通过与泛素连接酶的相互作用参与内质网应激相关性降解过程[15]。冷休克暴露期间,欧洲鲈HSP70基因表达水平升高,说明该基因负责防止蛋白质变性,聚集和协助错误折叠蛋白质的重新折叠,以维持细胞和蛋白质稳态,从而便于欧洲鲈可以更好地适应低温环境[16]。本研究中发现,低温暴露20 d后,低温组HSP20和HSP70基因表达上调,此类情况在栉孔扇贝低温胁迫时也存在[17]。推测HSP70基因被诱导表达,进而参与多种细胞过程,包括蛋白质折叠/去折叠、易位、靶向、降解和蛋白质复合物重塑,以维持细胞和蛋白质稳态,从而更好地应对冷应激。
硫氧还蛋白(thioredoxin,TRX)基因在转录水平调控蛋白质折叠和转运,从而维持内质网稳态[18]。本研究中发现,低温暴露20 d后,低温组TRX基因显著表达上调。前期研究发现,在日本沼虾铅胁迫浓度为26.26 μg/L时,Trx及TrxR mRNA的表达量明显下降,提示高浓度铅可通过诱导肝细胞 ROS水平升高,从而引发氧化应激,进而打破日本沼虾体内的氧化-抗氧化稳态[18]。此外,当铜浓度为1 μg/L的时候,中华绒螯蟹硫氧还蛋白的表达量增加,而在较低的条件下则无明显变化,提示高浓度铜胁迫下,硫氧还蛋白发挥催化二硫键形成、清除活性氧和减轻DNA损伤作用,从而调控线粒体氧化应激[19]。菲律宾蛤仔在铜和镉离子胁迫下,其肝脏中硫氧还蛋白1(Trx1)和硫氧还蛋白相关蛋白14(Trp14)的mRNA水平升高[20]。基于以上分析,推测TRX基因在菲律宾蛤仔适应低温暴露过程中发挥了重要的抗氧化功能,进而维持内质网稳态。
3.2 物质代谢
磷脂酰胆碱(phosphatidylcholine,PC)含量增加对维持细胞膜稳定性和通透性至关重要,而磷脂酶A和酰基转移酶3(phospholipase A and acyltransferase 3,PLAAT-3)可有效参与PC的生物合成[21]。在本研究中,低温暴露20、60 d后,低温组PLAAT-3基因均表达上调,同时该基因被富集到alpha-linolenic acid metabolism和linoleic acid metabolism KEGG通路。有研究发现,硬壳蛤中磷脂酶A1通过溶解因高盐胁迫而受损的细胞,从而维持细胞整体稳态[22]。推测菲律宾蛤仔可增强PC的合成来维持细胞膜稳定性和通透性,从而适应低温暴露过程。
酰基辅酶A去饱和酶(Acyl-CoA desaturase,ACD)参与长链不饱和脂肪酸的生物合成,而长链不饱和脂肪酸是膜脂的基本成分和细胞能量代谢的重要底物[23]。研究发现,在寒冷环境中,青海湖裸鲤脂肪酸代谢相关基因(酰基辅酶A合成酶长链基因和硬脂辅酶A去饱和酶基因)均高表达,提示这两个基因可能参与能量的产生,为青海湖裸鲤增加更多能量以维持其生理功能,进而适应寒冷环境[24]。随着温度的降低,红螯虾酰基辅酶AΔ9去饱和酶基因的表达逐渐增加,推测甲壳类动物对低温的耐受性与其酰基辅酶AΔ9去饱和酶活性和合成不饱和脂肪酸的能力有关[25]。本研究中发现,低温暴露20、60 d后,低温组ACD基因均表达上调,同时该基因被富集到biosynthesis of unsaturated fatty acids KEGG通路。在牡蛎低温暴露试验中,硬脂酰辅酶A去饱和酶基因也呈现高表达[26],表明双壳贝类可能通过提高调节膜组成和细胞能量代谢来适应低温暴露。
3.3 抗氧化
对于贝类而言,环境温度变化会加速其新陈代谢速率,导致机体ROS积累并激活抗氧化酶系统[27]。积累的ROS会造成蛋白质和DNA损伤,从而影响细胞的正常功能[28]。为控制ROS的大量生成和积累,贝类一般会利用抗氧化酶清除机体中过量的ROS、CAT、SOD、细胞色素P450(CYP450)、谷胱甘肽S转移酶(GST)及谷胱甘肽过氧化物酶(GPX)等[29]。已有研究发现,低温无水保活可显著提高缢蛏体内SOD活性、CAT活性及GSH含量,降低MDA与H2O2水平,降低肝糖原消耗及乳酸累积速度,保持缢蛏活性更强,进而有利于缢蛏长途运输[30]。此外,皱纹盘鲍血细胞中Cu/Zn-SOD mRNA相对表达量在低温胁迫后显著升高[31]。本研究中发现,低温组CYP450和GST基因表达水平在低温暴露20、60 d后均升高;低温暴露60 d后,低温组SOD和GPX基因均表达上调。在低温暴露60 d时,菲律宾蛤仔低温组SOD酶活力显著高于对照组,同时低温组MDA含量显著低于对照组,说明低温可诱导SOD基因高表达,进而清除机体过量的ROS,从而保护机体免受低温损伤。
3.4 免疫/抗凋亡
在外部病原体感染或环境因子影响下,贝类可以提高免疫相关基因表达,依靠先天免疫系统抵御入侵者[12]。已有研究发现,文蛤C型凝集素(C-type lectins,CLEC)作为模式识别受体,温度和盐度在一定范围内升高可诱导CLEC表达,进而调节免疫反应[32]。此外,虾夷扇贝在溶血弧菌细菌攻击和柴油燃料胁迫后,其血淋巴中CLEC的表达水平显著上调,表明CLEC可能参与细菌攻击和环境胁迫下的免疫反应[33]。牡蛎在暴露于低氧环境后,大多数基因的表达反应较小,但两个应激反应基因(即大防御素和 C 型凝集素)的表达较高,表明CLEC可能参与低氧胁迫下的免疫反应[34]。在本研究中发现,低温组CLEC和Galectin基因表达水平在低温暴露20、60 d后均升高;低温暴露60 d后,低温组PGRP基因表达显著增加,这说明菲律宾蛤仔可能会激活先天免疫系统来适应低温暴露。
除了先天免疫系统,贝类还可以依靠有效的抗凋亡系统来应对逆境胁迫[35]。凋亡抑制蛋白(inhibitors of apoptosis,IAP)和E3泛素蛋白连接酶(E3 ubiquitin-protein ligases,E3)是细胞程序性死亡和凋亡的关键调节因子[36-37]。IAP的抗凋亡功能,特别是对各种环境胁迫源的响应,已在许多贝类物种中得到证实。在热胁迫和冷胁迫下,长牡蛎的IAP表达水平显著上调[38];在缺氧暴露数天后,菲律宾蛤仔中IAP的表达水平也显著上调,说明贝类可以依靠抗凋亡系统来应对逆境胁迫[39]。研究发现,热敏感鲍鱼品系诱导更多泛素基因,及时降解不可修复的蛋白质[40]。此外,硬壳蛤E3泛素蛋白连接酶基因在热应激下表现出高表达,表明错误折叠的蛋白质可能通过泛素介导的蛋白水解被消除,以防止细胞毒性[35]。本研究中发现,低温暴露20、60 d后,低温组IAP和E3基因表达水平均显著升高,这表明菲律宾蛤仔可能通过调节其抗凋亡系统来应对低温暴露。
4 结论
1)在低温暴露下,菲律宾蛤仔鳃组织SOD活力先下降后上升,而MDA含量、CAT活力和T-AOC水平在60 d暴露过程中整体呈下降趋势。
2)通过对DEGs深入分析,发现蛤仔可能通过加强蛋白质合成来维持低温下细胞稳定性,通过加强PC合成维持细胞膜稳定性,同时诱导SOD基因高表达清除机体过量的ROS,从而保护机体免受低温损伤。
[1] 农业农村部渔业渔政管理局,全国水产技术推广总站,中国水产学会.2023中国渔业统计年鉴[M].北京:中国农业出版社,2023.Bureau of Fisheries,Ministry of Agriculture and Rural Affairs,National Fisheries Technology Extension Center,China Society of Fisheries.2023 China fishery statistical yearbook[M].Beijing:China Agriculture Press,2023.(in Chinese)
[2] 孙虎山,李光友.双壳贝类参与免疫防御的体液因子[J].海洋科学,2001,25(4):35-36.SUN H S,LI G Y.Humoral factors of bivalves participating in immunity defence[J].Marine Sciences,2001,25(4):35-36.(in Chinese)
[3] 钱佳慧,栗志民,申玉春,等.温度和盐度对华贵栉孔扇贝抗氧化酶活性的联合效应研究[J].南方水产科学,2015,11(6):49-57.QIAN J H,LI Z M,SHEN Y C,et al.Synergistic effect of temperature and salinity on antioxidant enzymes activities of Chlamys nobilis[J].South China Fisheries Science,2015,11(6):49-57.(in Chinese)
[4] 董莎莎,聂鸿涛,闫喜武.贝类低温胁迫响应机制研究进展[J].大连海洋大学学报,2019,34(3):457-462.DONG S S,NIE H T,YAN X W.Research progresses on mechanisms of cold stress responses in shellfish:a review[J].Journal of Dalian Ocean University,2019,34(3):457-462.(in Chinese)
[5] 李杰,孙溪蔓,张鹏,等.渐变低温对不同规格菲律宾蛤仔斑马蛤酶活性的影响[J].大连海洋大学学报,2018,33(2):217-222.LI J,SUN X M,ZHANG P,et al.Effect of gradual cooling on enzyme activity of Manila clam Ruditapes philippinarum with zebra-colored shell and different sizes[J].Journal of Dalian Ocean University,2018,33(2):217-222.(in Chinese)
[6] XU H,ZHANG D L,YU D H,et al.Molecular cloning and expression analysis of scd1 gene from large yellow croaker Larimichthys crocea under cold stress[J].Gene,2015,568(1):100-108.
[7] 李志强.温度变化对中华绒螯蟹生理功能的影响[D].哈尔滨:东北农业大学,2023.LI Z Q.Effects of temperature variationon physiological function of Eriocheir sinensis[D].Harbin:Northeast Agricultural University,2023.(in Chinese)
[8] 周康奇,韦孜娜,李哲,等.不同低温时间无水保活对中国圆田螺存活、营养组成、消化酶活性及抗氧化指标的影响[J].水产学报,2024,48(6):179-189.ZHOU K Q,WEI Z N,LI Z,et all.Effects of different times of low temperature anhydrous preservation on survival,nutritional composition,enzyme activities and antioxidant indexes of Cipangopaludina chinensis[J].Journal of Fisheries of China,2024,48(6):179-189.(in Chinese)
[9] ELLGAARD L,FRICKEL E M.Calnexin,calreticulin,and ERp57:teammates in glycoprotein folding[J].Cell Biochemistry and Biophysics,2003,39(3):223-247.
[10] HAN L S,QUAN Z J,WU Y L,et al.Expression regulation mechanisms of sea urchin (Strongylocentrotus intermedius) under the high temperature:new evidence for the miRNA-mRNA interaction involvement[J].Frontiers in Genetics,2022,13:876308.
[11] LEVIC D S,MINKEL J R,WANG W D,et al.Animal model of Sar1b deficiency presents lipid absorption deficits similar to Anderson disease[J].Journal of Molecular Medicine,2015,93(2):165-176.
[12] 井浩.低氧和高温胁迫对菲律宾蛤仔的组织结构、基因表达及能量代谢的影响研究[D].上海:上海海洋大学,2023.JING H.Effects of hypoxia and heat stress on tissue structure,gene expression and energy metabolism of clam Ruditapes philippinarum[D].Shanghai:Shanghai Ocean University,2023.(in Chinese)
[13] ALMANZA A,CARLESSO A,CHINTHA C,et al.Endoplasmic reticulum stress signalling-from basic mechanisms to clinical applications[J].The FEBS Journal,2019,286(2):241-278.
[14] NEEDHAM P G,GUERRIERO C J,BRODSKY J L.Chaperoning endoplasmic reticulum-associated degradation (ERAD) and protein conformational diseases[J].Cold Spring Harbor Perspectives in Biology,2019,11(8):a033928.
[15] GOLDBERG A L.Protein degradation and protection against misfolded or damaged proteins[J].Nature,2003,426(6968):895-899.
[16] ISLAM M J,KUNZMANN A,SLATER M J.Extreme winter cold-induced osmoregulatory,metabolic,and physiological responses in European seabass (Dicentrarchus labrax) acclimatized at different salinities[J].Science of the Total Environment,2021,771:145202.
[17] TAN K,ZHANG B,MA H Y,et al.Oxidative stress responses of golden and brown noble scallops Chlamys nobilis to acute cold stress[J].Fish &Shellfish Immunology,2019,95:349-356.
[18] 杨静云,周变华,王国永,等.硫氧还蛋白系统与病原体生物学功能的关系[J].动物医学进展,2021,42(10):105-108.YANG J Y,ZHOU B H,WANG G Y,et all.Relationship between thioredoxin system and biological function of pathogens[J].Progress in Veterinary Medicine,2021,42(10):105-108.(in Chinese)
[19] 孙曼曼.拟穴青蟹与中华绒螯蟹几个胁迫响应相关基因的克隆与表达分析[D].上海:上海海洋大学,2012.SUN M M.Cloning and expression profile of the responseto stress genes from mud crab,Scylla paramamosain and Eriocheir sinensis[D].Shanghai:Shanghai Ocean University,2012.(in Chinese)
[20] WANG Q,NING X X,CHEN L L,et al.Responses of thioredoxin 1 and thioredoxin-related protein 14 mRNAs to cadmium and copper stresses in Venerupis philippinarum[J].Comparative Biochemistry and Physiology Part C:Toxicology &Pharmacology,2011,154(3):154-160.
[21] UYAMA T,TSUBOI K,UEDA N.An involvement of phospholipase A/acyltransferase family proteins in peroxisome regulation and plasmalogen metabolism[J].FEBS Letters,2017,591(18):2745-2760.
[22] ZHOU C,XU L,SONG H,et al.Examination of the regulation of energy metabolism,antioxidant response,and ammonia detoxification in hard clam,Mercenaria mercenaria,under hypersalinity stress[J].Aquaculture,2023,563:738916.
[23] SMITH S,WITKOWSKI A,JOSHI A K.Structural and functional organization of the animal fatty acid synthase[J].Progress in Lipid Research,2003,42(4):289-317.
[24] LIU S J,LI X H,QI D L,et al.Genome-wide characterization of the Elovl gene family in Gymnocypris przewalskii and their potential roles in adaptation to cold temperature[J].Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology,2022,262:110759.
[25] WU D L,RAO Q X,CHENG L,et al.Cloning and characterisation of a Δ9 fatty acyl desaturase-like gene from the red claw crayfish (Cherax quadricarinatus) and its expression analysis under cold stress[J].Journal of Thermal Biology,2021,102:103122.
[26] WANG C G,LI A,CONG R H,et al.Cis-and trans-variations of stearoyl-CoA desaturase provide new insights into the mechanisms of diverged pattern of phenotypic plasticity for temperature adaptation in two congeneric oyster species[J].Molecular Biology and Evolution,2023,40(2):msad015.
[27] NAGALAKSHMI N,PRASAD M N.Copper-induced oxidative stress in Scenedesmus bijugatus:protective role of free radical scavengers[J].Bulletin of Environmental Contamination and Toxicology,1998,61(5):623-628.
[28] KONG X H,LV L Y,REN J F,et al.Comparative transcriptome analyses unravel the response to acute thermal stress in the razor clam,Sinonovacula constricta[J].Aquaculture Reports,2022,23:101079.
[29] BOURIOUG M,MAZZITELLI J Y,MARTY P,et al.Assessment of Lemna minor (duckweed) and Corbicula fluminea (freshwater clam) as potential indicators of contaminated aquatic ecosystems:responses to presence of psychoactive drug mixtures[J].Environmental Science and Pollution Research,2018,25(12):11192-11204.
[30] 方佳琪,张敏.低温无水保活对缢蛏活性氧代谢、糖原及乳酸的影响[J].安徽农业大学学报,2022,49(5):764-770.FANG J Q,ZHANG M.Effects of low-temperature waterless preservation on reactive oxygen species metabolism,glycogen and lactic acid of razor clam Sinonovacula constricta[J].Journal of Anhui Agricultural University,2022,49(5):764-770.(in Chinese)
[31] 姜娓娓,方建光,李加琦,等.温度胁迫对皱纹盘鲍生理和生化活动的影响[J].中国水产科学,2017,24(2):220-230.JIANG W W,FANG J G,LI J Q,et al.Effects of temperature change on physiological and biochemical activities of Haliotis discus Hannai Ino[J].Journal of Fishery Sciences of China,2017,24(2):220-230.(in Chinese)
[32] 李猛,周素明,刘璐,等.文蛤(Meretrix meretrix)C-型凝集素基因的分子克隆及表达分析[J].海洋与湖沼,2015,46(5):1186-1192.LI M,ZHOU S M,LIU L,et al.Molecular clone and expression of c-type lectin in Meretrix meretrix[J].Oceanologia Et Limnologia Sinica,2015,46(5):1186-1192.(in Chinese)
[33] MIZGINA T O,CHIKALOVETS I V,MOLCHANOVA V I,et al.Identification and characterization of a novel lectin from the clam Glycymeris yessoensis and its functional characterization under microbial stimulation and environmental stress[J].Marine Drugs,2021,19(9):474.
[34] FURR D,KETCHUM R N,PHIPPEN B L,et al.Physiological variation in response to Vibrio and hypoxia by aquacultured eastern oysters in the southeastern United States[J].Integrative and Comparative Biology,2021,61(5):1715-1729.
[35] HU Z,FENG J,SONG H,et al.Mechanisms of heat and hypoxia defense in hard clam:Insights from transcriptome analysis[J].Aquaculture,2022,549:737792.
[36] SONG H,GUO X M,SUN L N,et al.The hard clam genome reveals massive expansion and diversification of inhibitors of apoptosis in Bivalvia[J].BMC Biology,2021,19(1):15.
[37] BROEMER M,MEIER P.Ubiquitin-mediated regulation of apoptosis[J].Trends in Cell Biology,2009,19(3):130-140.
[38] ZHU Q H,ZHANG L L,LI L,et al.Expression characterization of stress genes under high and low temperature stresses in the Pacific oyster,Crassostrea gigas[J].Marine Biotechnology,2016,18(2):176-188.
[39] NIE H T,WANG H M,JIANG K Y,et al.Transcriptome analysis reveals differential immune related genes expression in Ruditapes philippinarum under hypoxia stress:potential HIF and NF-κB crosstalk in immune responses in clam[J].BMC Genomics,2020,21(1):318.
[40] CHEN N,HUANG Z K,LU C K,et al.Different transcriptomic responses to thermal stress in heat-tolerant and heat-sensitive Pacific abalones indicated by cardiac performance[J].Frontiers in Physiology,2019,9:1895.