褐藻多糖调控肠道微生态作用机制研究进展
章瑾1,王尚志1,杨明睿1,闫滨2*
(1.山东中医药大学 药学院,山东 济南 250355;2.山东中医药大学 中医学院,山东 济南 250355)
摘要:肠道作为机体最大的免疫器官,用以维持机体健康,而肠道微生态的失衡则易使机体出现代谢紊乱、免疫低下、炎症性肠病、病毒感染甚至组织癌变等情况。而褐藻来源的多糖可作为外源性益生元,通过保护肠道屏障、激活肠道免疫、调节肠道菌群和改变代谢产物构成等方式维持肠道稳态,从而维护机体健康。本文综述了褐藻多糖的化学组成和结构表征对肠道微生态的影响及其作用机制,并提出提高多糖得率和纯度、深入研究褐藻多糖构效关系与作用机制等未来发展建议,以期为推进褐藻多糖的开发与应用提供有益参考。
关键词:肠道微生态;肠道屏障;肠道菌群;免疫;褐藻多糖
DOI:10.16535/j.cnki.dlhyxb.2023-148
文章编号:2095-1388(2024)02-0349-11
中图分类号:S 917.3;R 285
文献标志码:A
收稿日期:2023-06-23
基金项目:国家“重大新药创制”科技重大专项(2014ZX09509001)
作者简介:章瑾(1999—),女,硕士研究生。E-mail:2021110268@sdutcm.edu.cn
通信作者:闫滨(1971—),男,博士,副教授。E-mail:robinyan2002@163.com
肠道作为机体最大的免疫器官,可通过分子相互作用选择性吸收水分和营养物质,并限制外源微生物的进入以维持肠道微生态平衡。有研究表明,肠道微生态的失衡不仅会引发肠道相关疾病,还会与远端器官相互作用影响外周循环,对肝脏、神经及内分泌系统造成损害,破坏机体免疫使其易受外来病原体的入侵,从而破坏机体健康平衡[1]。而褐藻多糖(brown algae polysaccharides)经肠道代谢分解后产生的寡糖及代谢物可为肠道菌群提供营养和能量,并调节菌群的结构组成,进而发挥调节免疫、抗炎、抗肿瘤及抗病毒等多种生物活性作用[2]。近年来,基于褐藻多糖调节肠道微生态进而预防或改善疾病的研究较为广泛,证实了褐藻多糖经肠道微生物降解后,可完善肠黏膜屏障,调控肠道免疫,干预肠道微生物及代谢产物的种类组成,发挥调节免疫、改善肠道菌群环境、抗炎、抗菌、抗肿瘤、降血糖血脂、调节情志和改善认知等功能[3-6]。除处于试验研究阶段的褐藻多糖外,已获批上市的甘露特钠胶囊(GV-971)还可改善阿尔兹海默病患者的认知功能[7]。基于褐藻多糖已有的研究成果,本文综述了褐藻多糖的化学组成和结构表征对肠道微生态的影响,并探讨了褐藻多糖调节微生态的作用机制,以期为褐藻多糖对肠道微生态的作用靶点研究提供新思路。
1 动物肠道微生态结构
动物肠道微生态主要由肠道上皮细胞、肠黏膜、肠道菌群及其代谢产物、免疫细胞构成。其中,肠黏膜可以防止病原体黏附到肠上皮细胞,保护肠上皮屏障[8];肠道菌群作为维持肠道微生态稳态的核心,可调节宿主生理和心理健康,维持动态平衡[9];肠道微生物的代谢产物如短链脂肪酸(short-chain fatty acids,SCFAs)等可被机体吸收,为细胞供能、增强紧密连接蛋白(tight junction,TJ)的表达,并抑制中性粒细胞和巨噬细胞释放促炎因子、激活肠道免疫;免疫细胞主要包括树突状细胞、巨噬细胞、T 细胞和B 细胞等淋巴细胞,其中超过80%的淋巴细胞位于肠黏膜上,约60%的辅助T细胞(CD4+T)位于与肠道相关的淋巴组织上,经外界刺激后可激发机体非特异性免疫,维护肠道稳态[10]。
肠道稳态随疾病阶段和侵袭部位的变化而变化。许多研究表明,肠道短期失调可能导致肠黏膜屏障受损,易引发胃肠道疾病,肠道长期失调则会通过与器官之间相互作用(如肠-肝、肠-肺和肠-脑轴等)引发慢性病[11-12]。广谱抗生素药物的滥用还会导致外来菌群的入侵,不仅使肠道菌群紊乱,引起肠道内较敏感的厌氧菌死亡,还会抑制肠上皮细胞蛋白的合成,破坏肠黏膜屏障,引发疾病感染或其他组织部位的病变[13]。此外,日常摄食习性与肠道干细胞和菌群组成也有关联,健康肠道的蠕动及其内容物的流动也会抑制体内微生物和病毒的过度生长[14-15]。因此,除通过增加有益的黏膜相关细菌类群来增强机体免疫外,还可以通过益生菌或微生物群的移植重建,校准肠道免疫稳态,从而改善或治疗疾病。如Shepherd等[16]通过对试验组小鼠喂食海藻多糖,利用多糖位点将目标菌属移植到具有不同肠道微生物群落的小鼠体内,成功实现了菌株的替换,改变了肠道菌属。Ai等[17]研究表明,海带来源的褐藻酸盐可以在人工肠道微环境中干预拟杆菌属中细金拟杆菌的丰度,从而改变了优势菌种。总之,肠道稳态与机体的健康密切相关,随着肠道微生态与相关疾病关系研究的深入,特征微生物组成或将成为疾病诊断治疗的方法之一。
2 褐藻多糖的化学组成和结构表征对肠道微生态的影响
海藻(Sargassaceae)和昆布(Laminaria thallus)等褐藻属海洋药物多被用于现代食药研究,其中,多糖类成分占藻类干质量的5%~20%[18],由于多糖不能被上消化道消化吸收,而是在肠道被肠道微生物降解,干预肠道微生物的种类组成,并产生大量低聚糖和SCFAs为机体供能,同时利用肠道与器官的相互作用进入外周循环,从而发挥调节免疫、改善肠菌群环境、抗炎、抗菌和抗肿瘤等生物活性作用[4-6,19-33](表1)。除具有上述生物活性功能外,褐藻多糖还可以通过激活肠道内神经元、改善血脑屏障等途径影响中枢系统,间接改善情感障碍类疾病[19]。因此,褐藻多糖的单糖组成、糖苷键构型、分子量和硫酸化修饰等表征因素都会影响肠道微生态的稳定。
表1 基于肠道微生态的褐藻属多糖生物活性
Tab.1 Biological activity of brown alga polysaccharides based on intestinal microecology
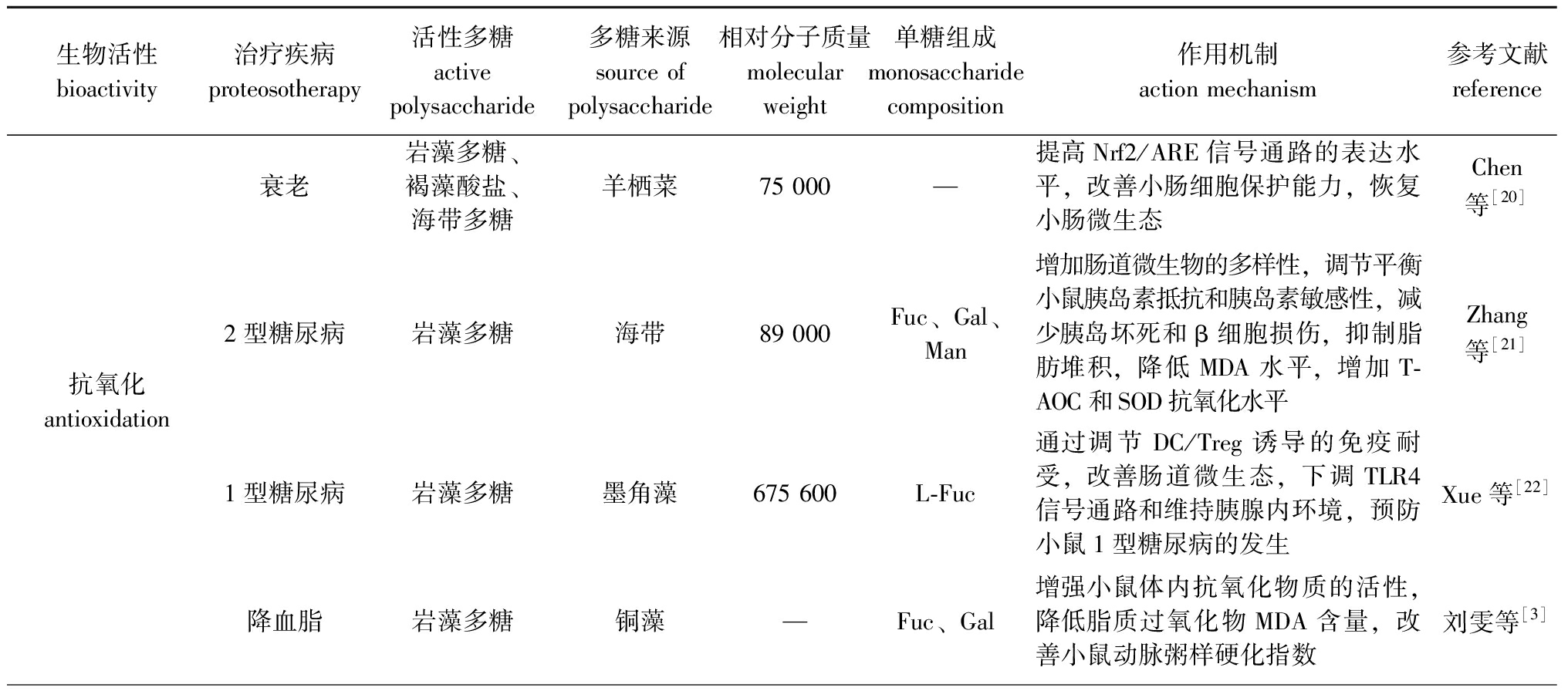
生物活性bioactivity治疗疾病proteosotherapy活性多糖active polysaccharide多糖来源source of polysaccharide相对分子质量molecular weight单糖组成monosaccharide composition作用机制action mechanism参考文献reference衰老岩藻多糖、褐藻酸盐、海带多糖羊栖菜75 000—提高Nrf2/ARE信号通路的表达水平,改善小肠细胞保护能力,恢复小肠微生态Chen等[20]抗氧化antioxidation2型糖尿病岩藻多糖 海带89 000Fuc、Gal、Man增加肠道微生物的多样性,调节平衡小鼠胰岛素抵抗和胰岛素敏感性,减少胰岛坏死和β细胞损伤,抑制脂肪堆积,降低MDA水平,增加T-AOC和SOD抗氧化水平Zhang等[21]1型糖尿病岩藻多糖墨角藻675 600L-Fuc通过调节DC/Treg诱导的免疫耐受,改善肠道微生态,下调TLR4信号通路和维持胰腺内环境,预防小鼠1型糖尿病的发生Xue等[22]降血脂岩藻多糖铜藻—Fuc、Gal增强小鼠体内抗氧化物质的活性,降低脂质过氧化物MDA含量,改善小鼠动脉粥样硬化指数刘雯等[3]
表1(续) 基于肠道微生态的褐藻属多糖生物活性
Tab.1(Cont.) Biological activity of brown alga polysaccharides based on intestinal microecology
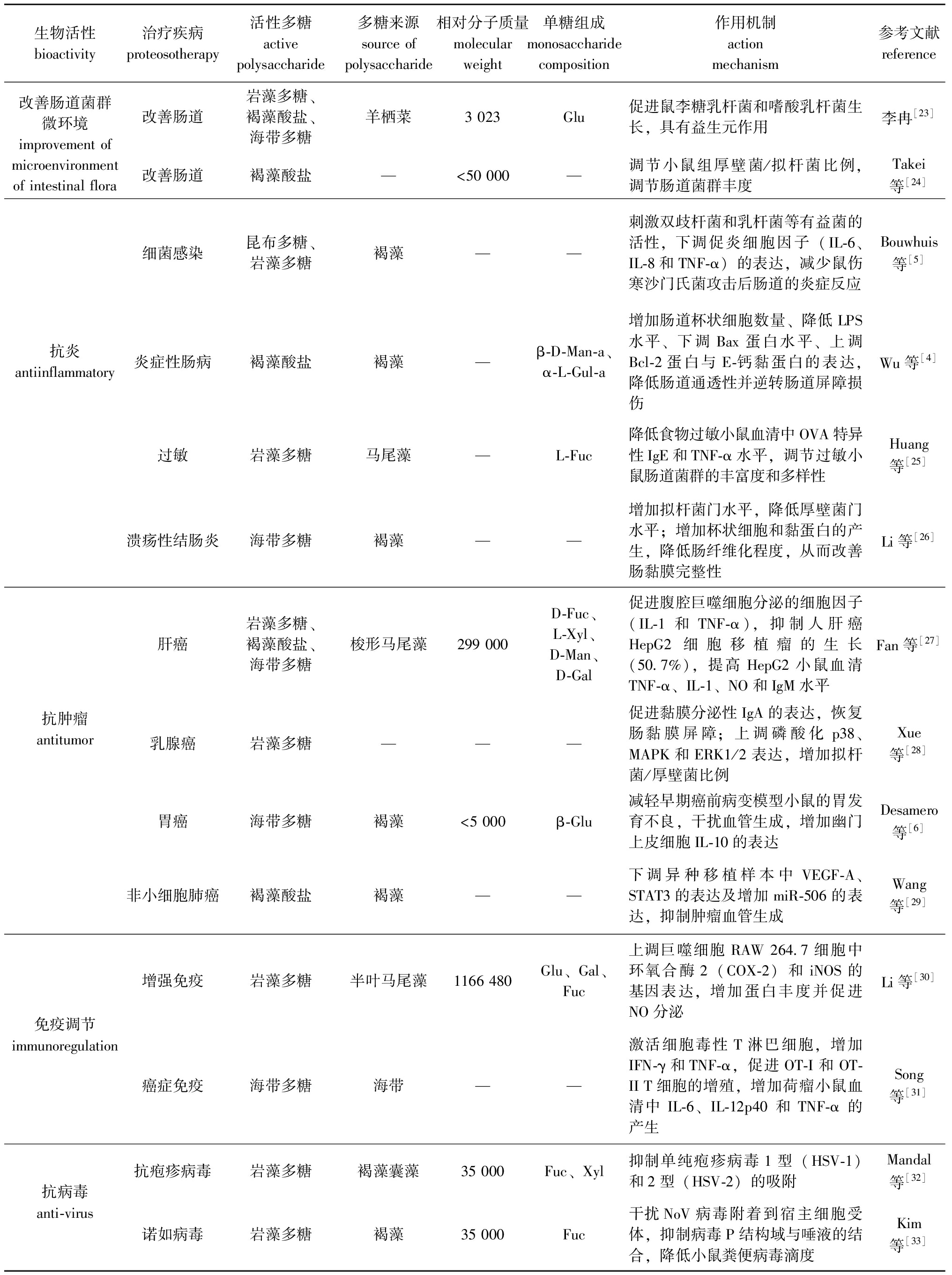
生物活性bioactivity治疗疾病proteosotherapy活性多糖active polysaccharide多糖来源source of polysaccharide相对分子质量molecular weight单糖组成monosaccharide composition作用机制action mechanism参考文献reference改善肠道菌群微环境 improvement of microenvironment of intestinal flora改善肠道岩藻多糖、褐藻酸盐、海带多糖羊栖菜3 023Glu促进鼠李糖乳杆菌和嗜酸乳杆菌生长,具有益生元作用李冉[23]改善肠道褐藻酸盐—<50 000—调节小鼠组厚壁菌/拟杆菌比例,调节肠道菌群丰度Takei等[24]细菌感染昆布多糖、岩藻多糖褐藻——刺激双歧杆菌和乳杆菌等有益菌的活性,下调促炎细胞因子(IL-6、IL-8和TNF-α)的表达,减少鼠伤寒沙门氏菌攻击后肠道的炎症反应Bouwhuis等[5]抗炎antiinflammatory炎症性肠病褐藻酸盐褐藻—β-D-Man-a、α-L-Gul-a 增加肠道杯状细胞数量、降低LPS水平、下调Bax蛋白水平、上调Bcl-2蛋白与E-钙黏蛋白的表达,降低肠道通透性并逆转肠道屏障损伤Wu等[4]过敏岩藻多糖马尾藻—L-Fuc降低食物过敏小鼠血清中OVA特异性IgE和TNF-α水平,调节过敏小鼠肠道菌群的丰富度和多样性Huang等[25]溃疡性结肠炎海带多糖褐藻——增加拟杆菌门水平,降低厚壁菌门水平;增加杯状细胞和黏蛋白的产生,降低肠纤维化程度,从而改善肠黏膜完整性Li等[26]抗肿瘤antitumor肝癌岩藻多糖、褐藻酸盐、海带多糖梭形马尾藻299 000D-Fuc、L-Xyl、D-Man、D-Gal促进腹腔巨噬细胞分泌的细胞因子(IL-1和TNF-α),抑制人肝癌HepG2细胞移植瘤的生长(50.7%),提高HepG2小鼠血清TNF-α、IL-1、NO和IgM水平Fan等[27]乳腺癌岩藻多糖———促进黏膜分泌性IgA的表达,恢复肠黏膜屏障;上调磷酸化p38、MAPK和ERK1/2表达,增加拟杆菌/厚壁菌比例Xue等[28]胃癌海带多糖褐藻<5 000β-Glu减轻早期癌前病变模型小鼠的胃发育不良,干扰血管生成,增加幽门上皮细胞IL-10的表达Desamero等[6]非小细胞肺癌褐藻酸盐褐藻——下调异种移植样本中VEGF-A、STAT3的表达及增加miR-506的表达,抑制肿瘤血管生成Wang等[29]免疫调节immunoregulation增强免疫岩藻多糖半叶马尾藻1166 480Glu、Gal、Fuc上调巨噬细胞RAW 264.7细胞中环氧合酶2(COX-2)和iNOS的基因表达,增加蛋白丰度并促进NO分泌Li等[30]癌症免疫海带多糖海带——激活细胞毒性T淋巴细胞,增加IFN-γ和TNF-α,促进OT-I和OT-II T细胞的增殖,增加荷瘤小鼠血清中 IL-6、IL-12p40 和 TNF-α 的产生Song等[31]抗病毒anti-virus抗疱疹病毒岩藻多糖褐藻囊藻35 000Fuc、Xyl抑制单纯疱疹病毒1型(HSV-1)和2型(HSV-2)的吸附Mandal等[32]诺如病毒岩藻多糖褐藻35 000Fuc干扰NoV病毒附着到宿主细胞受体,抑制病毒P结构域与唾液的结合,降低小鼠粪便病毒滴度Kim等[33]
注:—代表文中未提及。
Note:— is not mentioned in the text.
2.1 单糖组成对肠道微生态的影响
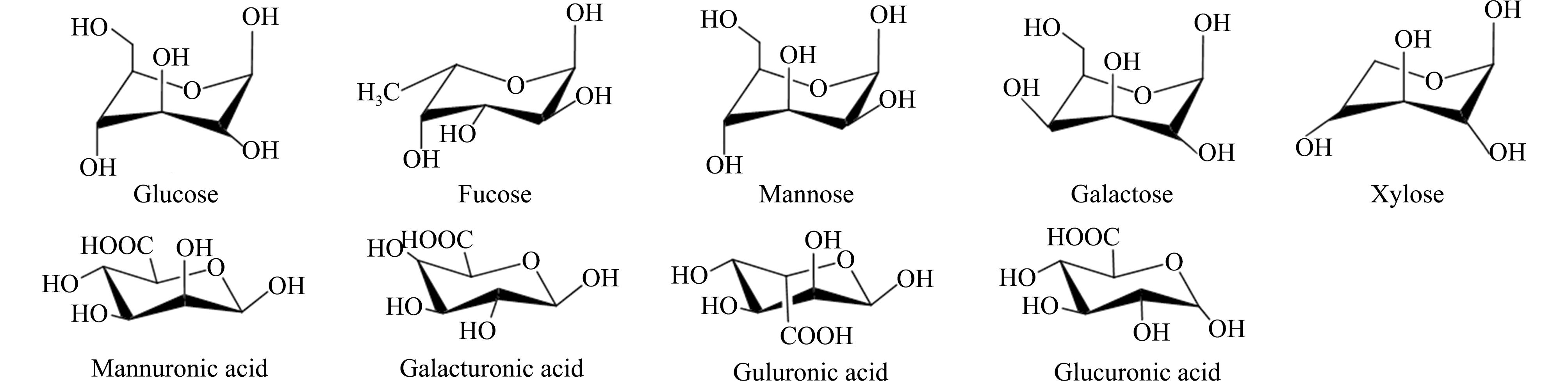
岩藻多糖(fucoidan)、海带多糖(laminaran)和褐藻酸钠(alginate)这3类褐藻多糖已被纳入疾病治疗的研究中,其单糖组成以岩藻糖(fucose)、葡萄糖(glucose)、甘露糖(mannose)、半乳糖(galactose)、木糖(xylose)、甘露糖醛酸(mannuronic acid)、半乳糖醛酸(galacturonic acid)、古罗糖醛酸(guluronic acid)和葡萄糖醛酸(glucuronic acid)等单糖及其衍生物为主(图1)。其中,单糖比例的不同对肠道的影响也各不相同,如葡萄糖含量较高的羊栖菜多糖(19.57%)对乳杆菌的促增殖作用要高于其他多糖,具有更好的益生元作用[34]。此外,单糖组成的复杂程度与生物活性也呈正相关,如Li等[35]提取的以葡萄糖(36.25%)、岩藻糖(18.31%)和甘露糖醛酸(17.3%)为主要成分的褐藻多糖,可通过修复肠道屏障降低肿瘤坏死因子-α(tumor necrosis factor-α,TNF-α)水平,促进丝裂原活化蛋白激酶(AMPK)磷酸化,减少肝脏脂质代谢积累从而降低肥胖风险。不同来源的岩藻多糖虽然单糖种类相似,但单糖比例及连接方式不同,也可能对肠道菌群的调节存在潜在影响,如泡叶藻和海带来源的岩藻多糖相比,前者能使盲肠微生物群落结构更加多样[36]。
2.2 糖苷键构型对肠道微生态的影响
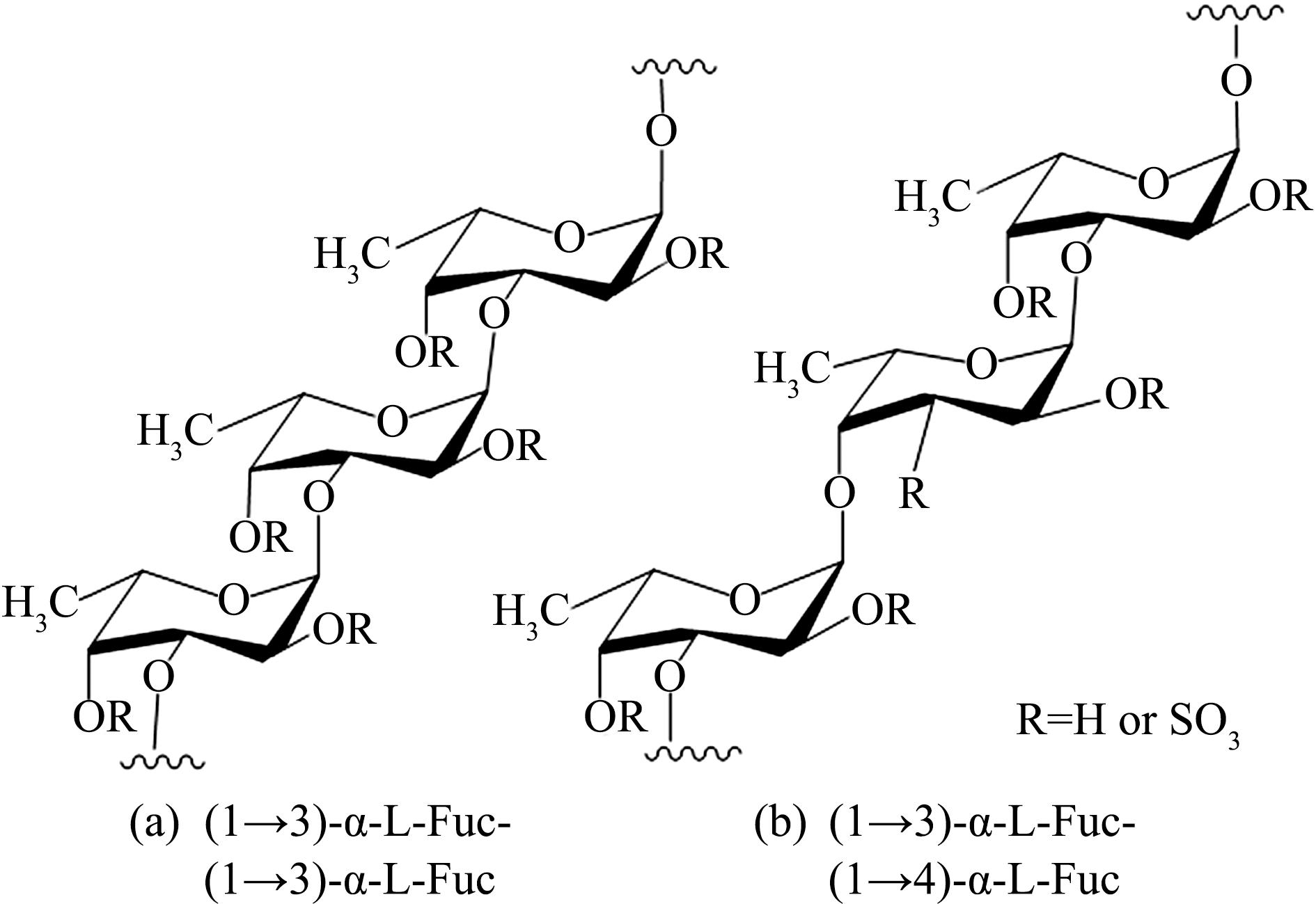
除单糖构成比例对肠道微生态影响外,多糖骨架对肠道菌群调节的机制也不完全相同。有研究认为,具有β-1,3糖苷键的多糖有明显的抗炎作用[37]。岩藻多糖主链以(1→3)-α-L-Fuc-(1→3)-α-L-Fuc(图2(a))和(1→3)-α-L-Fuc-(1→4)-α-L-Fuc(图2(b))两种构型存在,其硫酸基和单糖主要位于2,4或2,3链上[38-39],其机制是通过影响D-谷氨酰胺和D-谷氨酸代谢途径调节肠道菌群代谢,提高拟杆菌/厚壁菌的相对丰度,降低变形菌门的比例[40]。
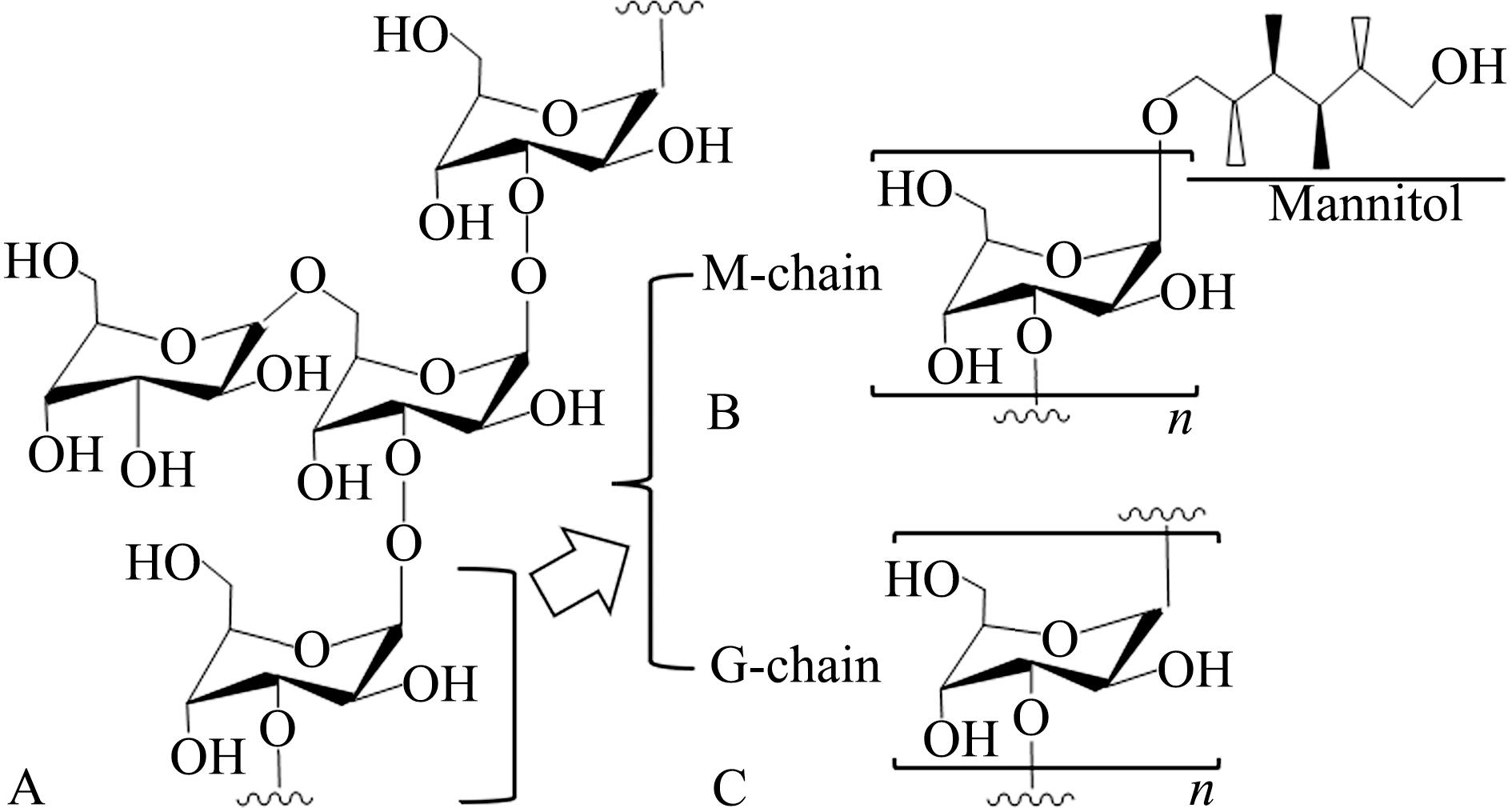
昆布多糖多以(1→3)-β-D-Glu为骨架,链内加部分(1→6)-β-D-Glu分支(图3(a)),且根据糖链还原端是否与甘露醇相连分为M链(图3(b))和G链(图3(c))[41]。昆布多糖作为一种线性多糖易被菌群代谢,一方面可通过增加拟杆菌门等益生菌丰度调节肠道菌群,另一方面通过调节代谢产物构成,如减少有害代谢物的产生,降低盲肠吲哚含量 [24,42]。
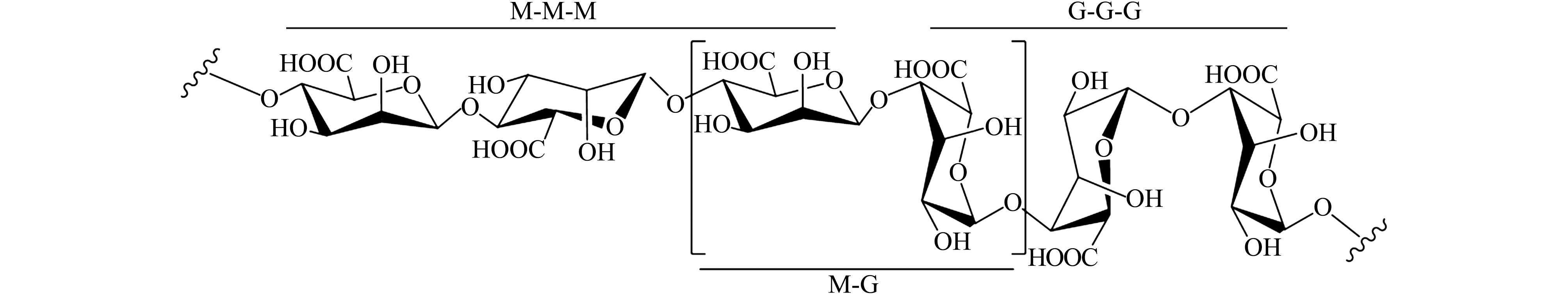
海藻酸钠构型以(1→4)-α-L-Gul acid-(1→4) Gul acid(G-G)、(1→4)-β-D-Mannuronic-(1→4)-β-D-Mannuronic(M-M)和(1→4)-α-L-Gul acid-(1→4)-β-D-Mannuronic(G-M)3种连接方式存在(图4),可被肠道微生物分解产生大量SCFAs,调节拟杆菌丰度,从而改善代谢型疾病(如糖尿病、胰岛素血症)等[43-44]。
综上,褐藻多糖的生物活性不仅与多糖结构有关,还与肠道微生物的构成相关,多糖结构和肠道微环境的不同,可能会导致多糖的酵解水平出现差异。因此,在后续研究中可比较不同结构褐藻多糖对肠道微生态差异的调节作用。
2.3 多糖分子量对肠道微生态的影响
褐藻多糖相对分子质量的大小也会影响其在肠道内的代谢吸收及屏障穿越修复能力。多数研究认为,低相对分子质量(<10 000)的褐藻多糖在肠道内的生物屏障穿越能力和修复活性更强,具有更高的吸收率[45-46]。研究发现,低分子量的褐藻多糖在不伤害人体健康的情况下,其代谢物丙酸盐、丁酸盐等SCFAs增加了61.85%,能更好地促进人胃肠道蠕动并抑制微生物的过度生长[47]。对比不同分子量的多糖发现,中低相对分子质量组(5 000~30 000)的岩藻多糖抗炎作用最强,可以有效抑制TNF-α、白细胞介素-1β(interleukin-1β,IL-1β)和白细胞介素-6 (interleukin-6,IL-6) 等促炎因子的产生[48]。进一步对中低分子量褐藻多糖分组后发现,相较于<6 000低相对分子质量的多糖,相对分子质量为26 700的褐藻多糖可使盲肠处拟杆菌门的相对丰度降低39.29%,更好地维持了肠道稳态[49]。但也有研究认为,中低分子量褐藻多糖可能会因失去支链导致生物活性降低,而高分子量多糖其硫酸根含量更高且在体内循环时间较长,在循环过程中可进入机体的其他器官组织,促进自然杀伤力细胞(NK)增殖,因此,对机体有更强的免疫激活及抗炎作用[50]。如从裙带菜(Undaria pinnatifida)和羊栖菜(Sargassum fusiforme)中分离得到的高相对分子质量的岩藻多糖(258 700),可降低Caco-2细胞中由过量活性氧造成的上皮细胞损伤引起的肠道炎症[50]。同时还发现,高分子量的岩藻多糖在血液中的平均停留时间(14.57 h)要高于低分子量的岩藻多糖(109 min),从而增加岩藻多糖在体内的生物利用度。从羊栖菜中提取的岩藻多糖(相对分子质量为707 000),可上调巨噬细胞促进一氧化氮(NO)的分泌,激活机体免疫[51]。综上,中低分子量的多糖因在体内能更好地被微生物群消化,其代谢产物的利用率要高于高分子量多糖,而在药物持续作用时长及整体免疫上,高分子量多糖更占优势,因此,不能仅以分子量大小作为生物活性高低的评判标准。
2.4 硫酸化修饰对肠道微生态的影响
硫酸化多糖可通过改善结肠上皮层的完整性,修护肠黏膜损伤,完善肠道屏障,发挥调节免疫、抗肿瘤、抗凝、抗炎、抗菌和抗病毒等生物活性作用[52]。一般来说,硫酸化多糖的生物活性与分子的硫酸化程度有关,含硫酸根的多糖在肠道中被微生物消化生成硫化氢(H2S),不仅可激活部分信号通路调节代谢,促进组织修复,维持心血管稳态,还可以屏蔽病毒受体的正电荷,防止病毒进入,抑制病毒与其靶细胞的结合[53-54]。如从海鞘藻(Gloiopeltis furcata)中提取的多糖可通过重塑肠道微生物群和黏蛋白O-聚糖之间的相互作用,减轻结肠黏膜损伤并促进益生菌生长[55];以硫酸化1,4-L-岩藻糖为主要单糖的褐藻多糖(66.08%),能与乳杆菌结合激活IL-6和IL-1β,增强巨噬细胞的非特异性免疫,使成年斑马鱼(Danio rerio)肠道淋巴细胞增加9.2%[56]。但需要注意,过量H2S经肠道细胞转化会生成硫代硫酸盐,进一步氧化为四硫酸盐,对肠上皮细胞造成损伤并促进沙门氏菌生长,从而造成炎症性腹泻[57]。
此外,其他功能性修饰的褐藻多糖也具有多种生物活性,如经硒化修饰后的褐藻酸盐能够降低血清和脑中 TNF-α和IL-6的产生,可作为补充药物治疗神经退行性疾病[58];羧酸化昆布多糖可调整肠道菌群结构,改善肠道受损后的肠黏膜完整性[26]。分子修饰已成为研究多糖构效关系的重要手段,也是发现和研制新型多糖类药物的重要途径,但在褐藻多糖中的研究较为少见。因此,在后续的研究中,可利用硫酸化、硒化、磷酸化、乙酰化和羧甲基化等功能性修饰,并根据褐藻多糖的分子量和空间构象探究其取代程度和取代位置,从而进一步增强其生物活性。但由于多糖活性基团含量越高,在肠道内代谢所需的菌群越复杂[50],因此,多糖修饰化程度还需考虑机体肠道菌群的丰度问题。
3 褐藻多糖调控肠道微生态的作用机制
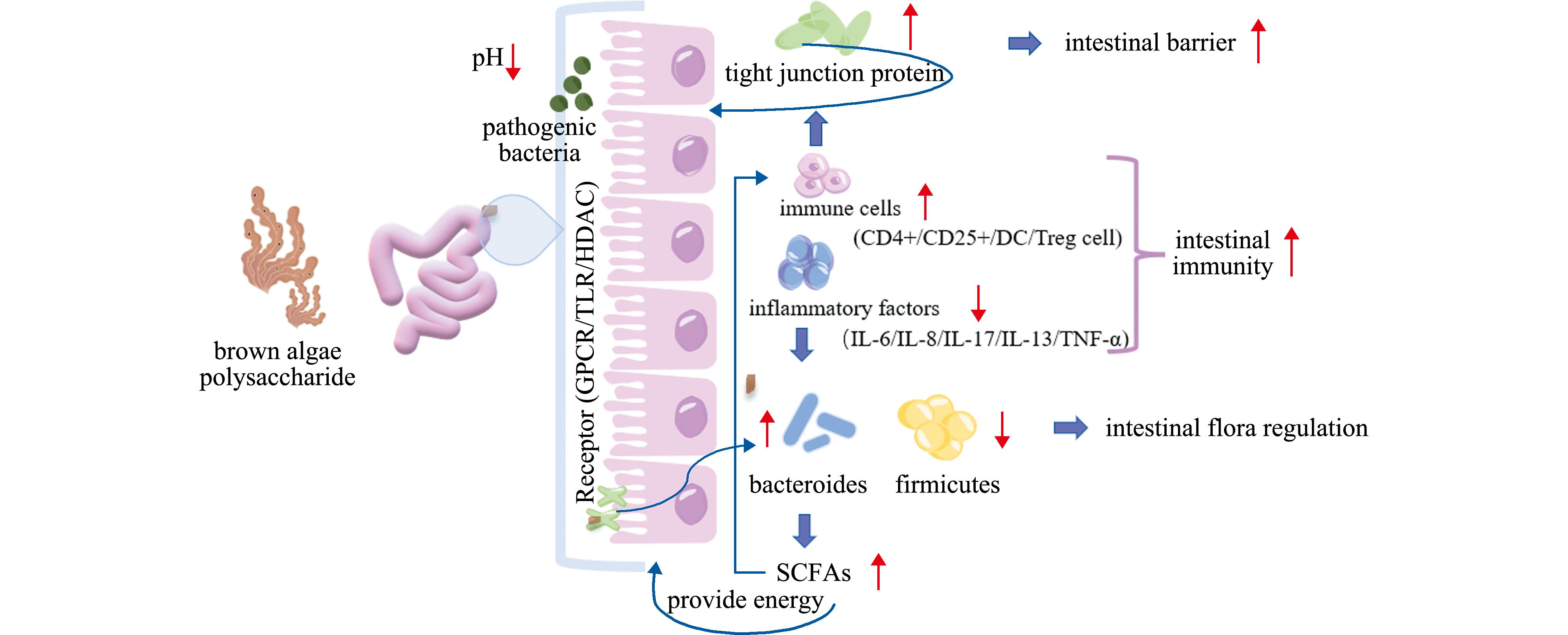
肠道是机体最大的代谢和免疫器官,参与全身疾病的调控,而褐藻多糖作为外源性益生元,可被肠道微生物降解为寡糖和相关代谢产物,保护肠道屏障并调节肠道菌群丰度,进而参与到肠道菌群对疾病的调控过程(图5)。褐藻多糖主要通过3个方面对肠道微生态进行调节:一是通过降低微环境的pH值抑制病原菌生长,防止病原体黏附到肠上皮细胞,促进TJ蛋白的表达从而保护肠上皮屏障;二是直接通过降低炎症因子的表达诱导免疫细胞成熟,激活免疫系统;三是作为配体与肠上皮细胞中表达的受体结合,如G 蛋白偶联受体(G protein-coupled receptors,GPCRs)、组蛋白去乙酰化酶(histone deacetylase,HDAC)和Toll 样受体(toll-like receptor,TLR)等,刺激有益菌生长并促进SCFAs 的产生,降低有害微生物的数量,调节菌群结构[8,59]。因此,褐藻多糖主要基于肠黏膜屏障、肠道免疫、肠道菌群及菌群代谢产物等方面对肠道微生态进行调控。
3.1 基于肠黏膜屏障调控
肠黏膜屏障被破坏易导致肠道通透性增加,使机体肠动力异常、菌群代谢紊乱,出现腹泻、便秘或急性肠炎等症状,还会通过与器官之间的相互作用(如肠-肝、肠-肺和肠-脑轴)引发炎症性肠病、肥胖、糖尿病、脂肪肝、高血脂及认知障碍等慢性疾病[4,11-12]。而褐藻多糖可以完善肠细胞膜蛋白功能,如促进TJ蛋白(包括occludin、ZO-1、Claudin-1等蛋白)和黏膜分泌性免疫球蛋白A的表达,修复肠黏膜屏障,降低肠道通透性,抵御肠道病毒和大肠杆菌等的入侵,预防因肠屏障破坏诱发的相关疾病[60]。如海藻多糖可通过抑制NF-κB信号通路、降低炎症因子表达,诱导小鼠肠黏膜Treg细胞分化,降低TNF-α表达水平,从而减轻肠道通透性并降低肠道炎症,改善由大肠杆菌诱导的肠屏障功能障碍和小鼠食物过敏症状[61];对溃疡性结肠炎小鼠模型喂食昆布多糖后发现,该多糖增加了小鼠肠道杯状细胞和黏蛋白的产生,从而降低了肠纤维化程度并维持肠黏膜完整性[26]。
癌症发生和治疗时,肠屏障均会受到破坏,导致肿瘤体积变大且预后效果差。如乳腺癌大鼠肠壁绒毛脱落,肠道通透性增加,但在摄入岩藻多糖后其肠道屏障功能基本恢复,抑瘤率可达49.2%,肿瘤潜伏期和肿瘤质量均较对照组缩短和减小[28]。此外,TFN-γ 也会通过降低肠内皮细胞的紧密连接减少结肠上皮细胞的通透性,增强内皮屏障功能[49]。在癌症患者临床治疗期间,常用化疗药环磷酰胺会损害肠绒毛细胞并下调TJ蛋白的表达,破坏肠道屏障,而褐藻多糖作为辅助药物可减缓该药的副作用,提高预后效果。如使用岩藻多糖干预化疗,可减轻患者肠道炎症并增加TJ蛋白的表达,调节绒毛长度与隐窝深度的比例,从而恢复肠黏膜屏障,最终减少化疗的副作用[62]。
综上,褐藻多糖作为益生元可维持肠黏膜屏障完整,使其免受到外来病原体的入侵,以及内部菌群结构和肠道免疫稳态的改变。
3.2 基于肠道免疫调控
肠道微环境的改变也会影响免疫系统稳态,而多糖可通过调节抗原呈递细胞、树突状细胞、巨噬细胞和多种细胞因子表现出较高的免疫调节能力[18]。如褐藻多糖可作为信号分子刺激抗原提呈细胞和上皮细胞识别受体,激活免疫细胞主动监测、识别和区分病原体在内的外部抗原,诱导肠道免疫并下调促炎相关因子(IL-6、IL-8和TNF-α)表达,从而减轻炎症反应,同时与微生物相互作用,共同维持肠道内环境的稳定[5,18]。如Ahmad等[63]用岩藻多糖(剂量为400 mg/kg)对急性结肠炎小鼠口服给药时发现,岩藻多糖可降低结肠NO、髓过氧化物酶、丙二醛的水平,减少炎症细胞浸润,进而恢复结肠长度并减少脾肿大。另有研究表明,岩藻多糖可通过TNF-α介导激活丝裂原活化蛋白激酶(MAPK-p38)、磷酸肌醇3激酶(PI3K)和糖原合酶激酶3 (GSK-3)参与诱导树突状细胞成熟[64]。此外,褐藻多糖还可与茶多酚协同增强机体免疫活性,通过对脂多糖诱导的RAW264.7细胞产生NO、ROS、IL-6和TNF-α,从而减轻肠道炎症[65]。
褐藻多糖除阻断G0/G1 细胞周期抑制癌细胞增殖外,还可增加外周血中NK和CD4+T细胞的比例,调节巨噬细胞和NK细胞释放的细胞因子激活免疫通路,从而影响肿瘤扩散[28,66]。在人肝癌小鼠模型中,Fan等[27]发现,用羊栖菜多糖(剂量为500 mg/mL)对HepG2细胞给药时,其细胞凋亡率最高(50.7%),HepG2荷瘤小鼠血清NO和IgM的浓度增加,其自身非特异性免疫增强。Tsai等[67]在转移性结直肠癌临床试验中也发现,受试者在摄入岩藻多糖(剂量为5 mg/kg)两周后,IL-1β、IL-6和TNF-α等促炎因子减少,疾病控制率增加了23.6%。
除肠道自身的炎症和癌症外,病毒感染也会削弱肠道屏障,改变有益菌群丰度,并通过肠-器官轴相互作用影响肠道、肺和肝脏等器官的正常功能,导致T细胞功能受损,从而降低机体抗病毒的免疫力[10,13]。而褐藻多糖一方面可通过增强内源性抗原呈递和共刺激功能促进树突状细胞成熟,从而恢复机体的非特异性免疫;另一方面还可与糖蛋白结合,抑制外源病毒的吸附和复制,共同发挥抗病毒作用[68-69]。目前,基于肠道微生态调控的角度探讨褐藻多糖对病毒性感染预防和治疗的研究较少,因此,在后续的研究中,可将肠道微生态纳入褐藻多糖抗病毒免疫的研究靶点。
3.3 基于肠道菌群调控
肠道微生物群的作用已被认为是疾病发病机制和体内平衡的关键,其中,有益微生物占优势是健康肠道微环境的特征之一,主要表现为90%的拟杆菌门和厚壁菌门,加之少量的变形菌和放线菌[70]。在肠道菌群的调节中,拟杆菌是降解多糖的主要菌群,主要以拟杆菌门、厚壁菌门、变形菌和双歧杆菌科等菌属的丰度作为指标,研究褐藻多糖对机体的情志、认知、代谢、炎症和癌变等相关疾病的调控[71]。
褐藻多糖可通过调节肠道菌群及其代谢产物的丰度改善肠道微环境,从而改善神经性疾病和代谢性疾病。如对模型小鼠喂食岩藻多糖发现,该多糖使厚壁菌丰度降低了7.32%,拟杆菌丰度增加了9.51%,并干预降低疣状微生物丰度,通过调节肠道菌群抑制小胶质细胞活化和炎症反应,共同减轻小鼠的抑郁行为[72]。除岩藻多糖外,褐藻酸钠、昆布多糖也可影响肠道菌群,调节脂多糖的表达,增加拟杆菌/厚壁菌的比例,降低幽门螺杆菌感染的风险,从而改善高血脂、高血糖等疾病。如利用褐藻酸钠和壳聚糖的黏附特性负载阿莫西林构建的纳米颗粒,可增强阿莫西林在胃部的保留时间,提高幽门螺杆菌的根除率,其治疗效果比标准三联疗法提高26%[73];对高脂饮食小鼠喂养褐藻多糖,通过代谢组学分析发现,该多糖可抑制肠道脂肪吸收,增加拟杆菌/厚壁菌比例,降低变形菌比例,使得肉毒碱和胆碱的代谢下降,减少胰岛坏死和β细胞损伤,达到降低血脂调节血糖的作用[74];来源于褐藻酸性低聚糖的临床药GV-971还可重建肠道微生物群,抑制大脑神经炎症,改善临床患者的认知功能[7]。此外,摄入褐藻多糖可以通过增加菌群丰度、促进代谢来逆转癌症恶化,主要表现为降低肿瘤质量、提高肿瘤抑制率和降低药物对机体的应激损伤[59]。研究发现,肠道菌群可通过影响乳腺癌患者雌激素的肠-肝循环和重吸收来调节机体雌激素水平,从而降低乳腺癌发病率[60]。
3.4 基于肠道菌群代谢产物调控
有研究认为,褐藻多糖还可通过调节肠道微生物群产生的功能性代谢物(如胆汁酸、三甲胺和SCFAs),提高新陈代谢,使脂质代谢正常化,减少氧化应激,从而降低心血管疾病(如动脉粥样硬化、高血压和心力衰竭等)风险[75]。SCFAs作为肠道微生物群发酵多糖的最终产物,已被证明是微生物群与宿主组织之间的纽带[17]。SCFAs主要包括乙酸盐、丙酸盐和丁酸盐等,可作为能量来源和信号分子参与机体的生命活动,具有抗炎、抗肿瘤、降低肥胖风险和保护肠道屏障等功能[76]。其中,乙酸盐进入外循环后可以穿过血脑屏障,通过抑制食欲调节神经肽的表达减少急性食物摄入,发挥抗肥胖作用,还可增强先天免疫反应抑制肠道感染;丁酸盐可为肠上皮细胞提供能量,调节结肠细胞的增殖和分化,维持肠道屏障的完整性,增强肠道免疫;丙酸盐可降低肝脏和血浆中的脂肪酸含量,刺激瘦素表达,并提高组织胰岛素敏感性,从而预防肥胖及糖尿病[77-78]。如在结肠癌试验中发现,岩藻多糖可增加肠道中的丙酸、异丁酸、丁酸和戊酸水平,降低模型结肠组织中TNF-α、IL-17、IL-23、IL-6和IL-1β等炎症因子的表达,并提高干扰素-γ、IL-4和IL-10的表达水平,从而降低肿瘤发生率,其抑瘤率为44.3%[66]。但不同微生物产生的SCFAs比例不同,因此,需联合肠道菌群的构成对激活的作用靶点及通路作进一步研究。
4 存在问题及展望
虽然现有研究表明,褐藻多糖可以通过调节肠道微生态呈现提高免疫、预防疾病和改善药物副作用等功能,但仍存在以下问题:一是基于褐藻多糖的研究多集中在生物活性的验证和肠道菌群相关组成的测试上,缺乏从肠道微生态整体角度探讨褐藻多糖对机体产生的整体影响;二是由于褐藻多糖的结构会受原料品种、生长环境和提取方法等客观因素的影响,其分子量、糖苷键构型及结合方式等不完全一致,其发挥的生物活性作用也各不相同;三是由于多糖的单糖构成及糖链类型并非单一不变,其调节肠道微生态的构效关系及作用机制尚未完全明确。因此,可以从以下几个方面开展后续研究。
4.1 提高褐藻多糖得率和纯度
褐藻属海洋植物众多,其提取纯化方式各不相同。当前褐藻多糖的提取方式主要为水提、酶提和超声波辅助等,并采用醇沉、柱层析和超滤法等方法纯化多糖。但褐藻多糖的得率较低,其过程对多糖的损耗较大。因此,可通过优化褐藻多糖提取方式、规范纯化流程,进而提高多糖得率和纯度,为后续多糖构效关系和生物活性研究奠定物质基础。
4.2 深入开展褐藻多糖的构效关系和作用机制研究
由于肠道微生物的复杂性和多糖难被消化吸收的特性,当前褐藻多糖研究多集中在体外及动物模型试验中,而动物模型在肠道结构功能、菌群组成和营养代谢等方面仍然与人类存在差异,从而导致褐藻多糖在人类机体内的药代动力和组织分布研究仍存在空白。因此,可根据褐藻多糖的单糖组成、分子量和糖苷键构型等可知因素,在更高等的动物模型上明确褐藻多糖通过肠道微生态发挥生物活性作用的物质结构基础及相关靶点通路,深入探究褐藻多糖结构与生物活性的关系,并综合考虑肠道微生态的个体差异化,以提高褐藻多糖的生物利用度。
4.3 推进褐藻多糖的开发与应用
褐藻多糖作为一种丰富的海洋资源,具有量大、价廉和易得的优势,其生物活性也较为多样,具有较高的开发利用价值。目前,褐藻多糖研究主要集中在食品、药品及保健品等领域,主要作为膳食益生元或药物辅助剂,用于提高机体免疫,改善健康状况。综上所述,在明确褐藻多糖构效关系和作用机制的基础上,应继续完善褐藻多糖的研究和开发,拓宽海藻资源,提升褐藻多糖相关功能性食品及新药的研发水平。
参考文献:
[1] SUN T,XUE M L,YANG J,et al.Metabolic regulation mechanism of fucoidan via intestinal microecology in diseases[J].Journal of the Science of Food and Agriculture,2021,101(11):4456-4463.
[2] YU Y,SHEN M Y,SONG Q Q,et al.Biological activities and pharmaceutical applications of polysaccharide from natural resources:a review[J].Carbohydrate Polymers,2018,183:91-101.
[3] 刘雯,车心怡,马志超,等.铜藻岩藻聚糖硫酸酯的结构组成及降血脂作用[J].大连海洋大学学报,2023,38(2):323-330. LIU W,CHE X Y,MA Z C,et al.Structural composition and hypolipidemic effect of fucoidan from Sargassum horneri[J].Journal of Dalian Ocean University,2023,38(2):323-330.(in Chinese)
[4] WU A X,GAO Y,KAN R T,et al.Alginate oligosaccharides prevent dextran-sulfate-sodium-induced ulcerative colitis via enhancing intestinal barrier function and modulating gut microbiota[J].Foods,2023,12(1):220.
[5] BOUWHUIS M A,MCDONNELL M J,SWEENEY T,et al.Seaweed extracts and galacto-oligosaccharides improve intestinal health in pigs following Salmonella typhimurium challenge[J].Animal:The International Journal of Animal Bioscience,2017,11(9):1488-1496.
[6] DESAMERO M J,KAKUTA S,CHAMBERS J K,et al.Orally administered brown seaweed-derived β-glucan effectively restrained development of gastric dysplasia in A4gnt KO mice that spontaneously develop gastric adenocarcinoma[J].International Immunopharmacology,2018,60:211-220.
[7] XIAO S F,CHAN P,WANG T,et al.A 36-week multicenter,randomized,double-blind,placebo-controlled,parallel-group,phase 3 clinical trial of sodium oligomannate for mild-to-moderate Alzheimer’s dementia[J].Alzheimer’s Research &Therapy,2021,13(1):62.
[8] DO M H,SEO Y S,PARK H Y.Polysaccharides:bowel health and gut microbiota[J].Critical Reviews in Food Science and Nutrition,2021,61(7):1212-1224.
[9] LORDAN C,THAPA D,ROSS R P,et al.Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components[J].Gut Microbes,2020,11(1):1-20.
[10] GENG S T,ZHANG Z Y,WANG Y X,et al.Regulation of gut microbiota on immune reconstitution in patients with acquired immunodeficiency syndrome[J].Frontiers in Microbiology,2020,11:594820.
[11] LIU J,WU S Y,CHENG Y,et al.Sargassum fusiforme alginate relieves hyperglycemia and modulates intestinal microbiota and metabolites in type 2 diabetic mice[J].Nutrients,2021,13(8):2887.
[12] BAO Y F,DONG C,JI J,et al.Dysregulation of gut microbiome is linked to disease activity of rheumatic diseases[J].Clinical Rheumatology,2020,39(9):2523-2528.
[13] GUO W N,ZHOU X,LI X R,et al.Depletion of gut microbiota impairs gut barrier function and antiviral immune defense in the liver[J].Frontiers in Immunology,2021,12:636803.
[14] KISHIDA K,PEARCE S C,YU S Y,et al.Nutrient sensing by absorptive and secretory progenies of small intestinal stem cells[J].American Journal of Physiology Gastrointestinal and Liver Physiology,2017,312(6):G592-G605.
[15] DE GRAAF M,VILLABRUNA N,KOOPMANS M P.Capturing norovirus transmission[J].Current Opinion in Virology,2017,22:64-70.
[16] SHEPHERD E S,DELOACHE W C,PRUSS K M,et al.An exclusive metabolic niche enables strain engraftment in the gut microbiota[J].Nature,2018,557(7705):434-438.
[17] AI C Q,JIANG P R,LIU Y L,et al.The specific use of alginate from Laminaria japonica by Bacteroides species determined its modulation of the Bacteroides community[J].Food &Function,2019,10(7):4304-4314.
[18] YANG J Y,LIM S Y.Fucoidans and bowel health[J].Marine Drugs,2021,19(8):436.
[19] SHARON G,SAMPSON T R,GESCHWIND D H,et al.The central nervous system and the gut microbiome[J].Cell,2016,167(4):915-932.
[20] CHEN P,YANG S,HU C,et al.Sargassum fusiforme polysaccharide rejuvenates the small intestine in mice through altering its physiology and gut microbiota composition[J].Current Molecular Medicine,2017,17(5):350-358.
[21] ZHANG C X,JIA J H,ZHANG P P,et al.Fucoidan from Laminaria japonica ameliorates type 2 diabetes mellitus in association with modulation of gut microbiota and metabolites in streptozocin-treated mice[J].Foods,2022,12(1):33.
[22] XUE M L,LIANG H,JI X Q,et al.Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways,regulation DC/Treg induced immune tolerance and improving gut microecology[J].Nutrition &Metabolism,2019,16:87.
[23] 李苒.羊栖菜低聚糖的制备及其理化性质研究[D].上海:华东理工大学,2019. LI R.Preparation and physicochemical properties of oligosaccharide from Sargassum fusiformis[D].Shanghai:East China University of Science and Technology,2019.(in Chinese)
[24] TAKEI M N,KUDA T,TANIGUCHI M,et al.Detection and isolation of low molecular weight alginate-and laminaran-susceptible gut indigenous bacteria from ICR mice[J].Carbohydrate Polymers,2020,238:116205.
[25] HUANG L,ZENG Q H,ZHANG Y D,et al.Effects of fucoidans and alginates from Sargassum graminifolium on allergic symptoms and intestinal microbiota in mice with OVA-induced food allergy[J].Food &Function,2022,13(12):6702-6715.
[26] LI Y F,UDAYAKUMAR V,SATHUVAN M,et al.Effects of laminarin zwitterionic carboxylate and sulfonate on the intestinal barrier function and gut microbiota[J].Carbohydrate Polymers,2022,278:118898.
[27] FAN S R,ZHANG J F,NIE W J,et al.Antitumor effects of polysaccharide from Sargassum fusiforme against human hepatocellular carcinoma HepG2 cells[J].Food and Chemical Toxicology:An International Journal Published for the British Industrial Biological Research Association,2017,102:53-62.
[28] XUE M L,JI X Q,LIANG H,et al.The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer[J].Food &Function,2018,9(2):1214-1223.
[29] WANG K P,WANG B,WANG Z Q,et al.Alginic acid inhibits non-small cell lung cancer-induced angiogenesis via activating miR-506 expression[J].Journal of Natural Medicines,2021,75(3):553-564.
[30] LI R,ZHOU Q L,CHEN S T,et al.Chemical characterization and immunomodulatory activity of fucoidan from Sargassum hemiphyllum[J].Marine Drugs,2022,21(1):18.
[31] SONG K,XU L,ZHANG W,et al.Laminarin promotes anti-cancer immunity by the maturation of dendritic cells[J].Oncotarget,2017,8(24):38554-38567.
[32] MANDAL P,MATEU C G,CHATTOPADHYAY K,et al.Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica[J].Antiviral Chemistry &Chemotherapy,2007,18(3):153-162.
[33] KIM H,LIM C Y,LEE D B,et al.Inhibitory effects of Laminaria japonica fucoidans against noroviruses[J].Viruses,2020,12(9):997.
[34] 孔秋红,张瑞芬,曾新安,等.不同方法提取的羊栖菜多糖理化性质及益生活性[J].现代食品科技,2021,37(5):123-129. KONG Q H,ZHANG R F, ZENG X A,et al.Physicochemical properties and prebiotic activity of Sargassum fusiforme polysaccharides obtained by different extraction methods[J].Modern Food Science and Technology,2021,37(5):123-129.(in Chinese)
[35] LI N N,FU X D,XIAO M S,et al.Enzymatic preparation of a low-molecular-weight polysaccharide rich in uronic acid from the seaweed Laminaria japonica and evaluation of its hypolipidemic effect in mice[J].Food &Function,2020,11(3):2395-2405.
[36] SHANG Q S,SHAN X D,CAI C,et al.Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae[J].Food &Function,2016,7(7):3224-3232.
[37] WANG Q,LIU F,CHEN X X,et al.Effects of the polysaccharide SPS-3-1 purified from Spirulina on barrier integrity and proliferation of Caco-2 cells[J].International Journal of Biological Macromolecules,2020,163:279-287.
[38] BOUISSIL S,PIERRE G,ALAOUI-TALIBI Z E,et al.Applications of algal polysaccharides and derivatives in therapeutic and agricultural fields[J].Current Pharmaceutical Design,2019,25(11):1187-1199.
[39] VAN WEELDEN G,BOBINSKI M,OK A K,et al.Fucoidan structure and activity in relation to anti-cancer mechanisms[J].Marine Drugs,2019,17(1):32.
A K,et al.Fucoidan structure and activity in relation to anti-cancer mechanisms[J].Marine Drugs,2019,17(1):32.
[40] CHEN A J,LIU Y T,ZHANG T,et al.Chain conformation,mucoadhesive properties of fucoidan in the gastrointestinal tract and its effects on the gut microbiota[J].Carbohydrate Polymers,2023,304:120460.
[41] USOLTSEVA R V,ANASTYUK S D,SHEVCHENKO N M,et al.Polysaccharides from brown algae Sargassum duplicatum:the structure and anticancer activity in vitro[J].Carbohydrate Polymers,2017,175:547-556.
[42] NAKATA T,KYOUI D,TAKAHASHI H,et al.Inhibitory effects of laminaran and alginate on production of putrefactive compounds from soy protein by intestinal microbiota in vitro and in rats[J].Carbohydrate Polymers,2016,143:61-69.
[43] SANCHEZ-BALLESTER N M,BATAILLE B,SOULAIROL I.Sodium alginate and alginic acid as pharmaceutical excipients for tablet formulation:structure-function relationship[J].Carbohydrate Polymers,2021,270:118399.
[44] YANG Y,LIANG M N,OUYANG D,et al.Research progress on the protective effect of brown algae-derived polysaccharides on metabolic diseases and intestinal barrier injury[J].International Journal of Molecular Sciences,2022,23(18):10784.
[45] YOO H J,YOU D J,LEE K W.Characterization and immunomodulatory effects of high molecular weight fucoidan fraction from the sporophyll of Undaria pinnatifida in cyclophosphamide-induced immunosuppressed mice[J].Marine Drugs,2019,17(8):447.
[46] SUN T,XUE M L,YANG J,et al.Metabolic regulation mechanism of fucoidan via intestinal microecology in diseases[J].Journal of the Science of Food and Agriculture,2021,101(11):4456-4463.
[47] BANNON C D,ECKENBERGER J,SNELLING W J,et al.Low-molecular-weight seaweed-derived polysaccharides lead to increased faecal bulk but do not alter human gut health markers[J].Foods,2021,10(12):2988.
[48] AHMAD T,EAPEN M S,ISHAQ M,et al.Anti-inflammatory activity of fucoidan extracts in vitro[J].Marine Drugs,2021,19(12):702.
[49] LUO J M,WANG Z,FAN B,et al.A comparative study of the effects of different fucoidans on cefoperazone-induced gut microbiota disturbance and intestinal inflammation[J].Food &Function,2021,12(19):9087-9097.
[50] LIM J M,YOO H J,LEE K W.High molecular weight fucoidan restores intestinal integrity by regulating inflammation and tight junction loss induced by methylglyoxal-derived hydroimidazolone-1[J].Marine Drugs,2022,20(9):580.
[51] 刘雪,王桂宏,赵福江,等.羊栖菜褐藻糖胶的结构表征及其抗氧化活性[J].食品工业科技,2019,40(3):79-84. LIU X,WANG G H,ZHAO F J,et al.Structural characterization and antioxidant activities of fucoidan from Sargassum fusiforme[J].Science and Technology of Food Industry,2019,40(3):79-84.(in Chinese)
[52] MAZEPA E,BISCAIA S M P,BELLAN D L,et al.Structural characteristics of native and chemically sulfated polysaccharides from seaweed and their antimelanoma effects[J].Carbohydrate Polymers,2022,289:119436.
[53] LI Z,POLHEMUS D J,LEFER D J.Evolution of hydrogen sulfide therapeutics to treat cardiovascular disease[J].Circulation Research,2018,123(5):590-600.
[54] JABEEN M,DUTOT M,FAGON R,et al.Seaweed sulfated polysaccharides against respiratory viral infections[J].Pharmaceutics,2021,13(5):733.
[55] PAN L,FU T Y,CHENG H,et al.Polysaccharide from edible alga Gloiopeltis furcata attenuates intestinal mucosal damage by therapeutically remodeling the interactions between gut microbiota and mucin O-glycans[J].Carbohydrate Polymers,2022,278:118921.
[56] KIM Y S,HWANG J,LEE S G,et al.Structural characteristics of sulfated polysaccharides from Sargassum horneri and immune-enhancing activity of polysaccharides combined with lactic acid bacteria[J].Food &Function,2022,13(15):8214-8227.
[57] PEREZ-LOPEZ A,BEHNSEN J,NUCCIO S P,et al.Mucosal immunity to pathogenic intestinal bacteria[J].Nature Reviews Immunology,2016,16(3):135-148.
[58] BI D C,LAI Q X,LI X F,et al.Neuroimmunoregulatory potential of seleno-polymannuronate derived from alginate in lipopolysaccharide-stimulated BV2 microglia[J].Food Hydrocolloids,2019,87:925-932.
[59] LIU L Q,LI M Z,YU M L,et al.Natural polysaccharides exhibit anti-tumor activity by targeting gut microbiota[J].International Journal of Biological Macromolecules,2019,121:743-751.
[60] QIN J J,LI R Q,RAES J,et al.A human gut microbial gene catalogue established by metagenomic sequencing[J].Nature,2010,464(7285):59-65.
[61] GUO X B,CHEN J,YANG J,et al.Seaweed polysaccharide mitigates intestinal barrier dysfunction induced by enterotoxigenic Escherichia coli through NF-κB pathway suppression in porcine intestinal epithelial cells[J].Journal of Animal Physiology and Animal Nutrition,2021,105(6):1063-1074.
[62] SHI H J,CHANG Y G,GAO Y,et al.Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide[J].Food &Function,2017,8(9):3383-3393.
[63] AHMAD T,ISHAQ M,KARPINIEC S,et al.Oral Macrocystis pyrifera fucoidan administration exhibits anti-inflammatory and antioxidant properties and improves DSS-induced colitis in C57BL/6J mice[J].Pharmaceutics,2022,14(11):2383.
[64] KHIL’CHENKO S R,ZAPOROZHETS T S,SHEVCHENKO N M,et al.Immunostimulatory activity of fucoidan from the brown alga Fucus evanescens:role of sulfates and acetates[J].Journal of Carbohydrate Chemistry,2011,30(4/5/6):291-305.
[65] SHEN S Q,YANG W Q,LI L J,et al.In vitro fermentation of seaweed polysaccharides and tea polyphenol blends by human intestinal flora and their effects on intestinal inflammation[J].Food &Function,2023,14(2):1133-1147.
[66] XUE M L,LIANG H,JI X Q,et al.Effects of fucoidan on gut flora and tumor prevention in 1,2-dimethylhydrazine-induced colorectal carcinogenesis[J].The Journal of Nutritional Biochemistry,2020,82:108396.
[67] TSAI H L,TAI C J,HUANG C W,et al.Efficacy of low-molecular-weight fucoidan as a supplemental therapy in metastatic colorectal cancer patients:a double-blind randomized controlled trial[J].Marine Drugs,2017,15(4):122.
[68] PRADHAN B,NAYAK R,PATRA S,et al.A state-of-the-art review on fucoidan as an antiviral agent to combat viral infections[J].Carbohydrate Polymers,2022,291:119551.
[69] SUN Q L,LI Y,NI L Q,et al.Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum[J].Carbohydrate Polymers,2020,229:115487.
[70] VAN TREUREN W,DODD D.Microbial contribution to the human metabolome:implications for health and disease[J].Annual Review of Pathology,2020,15:345-369.
[71] 张玉姣,孙晓娜,田伟功,等.岩藻多糖及其降解物在小鼠肠道中的代谢及对肠道代谢产物的影响[J].现代食品科技,2022,38(12):26-33. ZHANG Y J,SUN X N,TIAN W G,et al.Metabolism of fucoidan and its degradation products in themurine gut and their effects on gut metabolites[J].Modern Food Science and Technology,2022,38(12):26-33.(in Chinese)
[72] XUE M,TENG X,LIANG H,et al.Neuroprotective effect of fucoidan by regulating gut-microbiota-brain axis in alcohol withdrawal mice[J].Journal of Functional Foods,2021,86: 104726.
[73] YAO C J,YANG S J,HUANG C H,et al.Retention time extended by nanoparticles improves the eradication of highly antibiotic-resistant Helicobacter pylori[J].Pharmaceutics,2022,14(10):2117.
[74] MURAKAMI S,HIRAZAWA C,OHYA T,et al.The edible brown seaweed Sargassum horneri (Turner) C.agardh ameliorates high-fat diet-induced obesity,diabetes,and hepatic steatosis in mice[J].Nutrients,2021,13(2):551.
[75] IKEDA T,NISHIDA A,YAMANO M,et al.Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic,immune,and neurological diseases[J].Pharmacology &Therapeutics,2022,239:108273.
[76] WEI B,ZHANG B,DU A Q,et al.Saccharina japonica Fucan suppresses high fat diet-induced obesity and enriches fucoidan-degrading gut bacteria[J].Carbohydrate Polymers,2022,290:119411.
[77] YUE B J,ZONG G F,TAO R Z,et al.Crosstalk between traditional Chinese medicine-derived polysaccharides and the gut microbiota:a new perspective to understand traditional Chinese medicine[J].Phytotherapy Research:PTR,2022,36(11):4125-4138.
[78] LI X,XIN S J,ZHENG X Q,et al.Inhibition of the occurrence and development of inflammation-related colorectal cancer by fucoidan extracted from Sargassum fusiforme[J].Journal of Agricultural and Food Chemistry,2022,70(30):9463-9476.
Research progress on effect of brown alga polysaccharides on regulation mechanism of intestinal microecology: a review
ZHANG Jin1,WANG Shangzhi1,YANG Mingrui1,YAN Bin2*
(1.School of Pharmacy,Shandong University of Traditional Chinese Medicine,Jinan 250355,China;2.College of Traditional Chinese Medicine,Shandong University of Traditional Chinese Medicine,Jinan 250355,China)
Abstract: Intestinal tract as the largest immune organ in a body is used to maintain the health, but the imbalance of intestinal microecology is easy to cause metabolic disorders, immunosuppression, inflammatory bowel disease, viral infection and even tissue canceration. Polysaccharides derived from brown algae can be used as exogenous prebiotics by protecting intestinal barrier, activating intestinal immunity, regulating intestinal flora and changing the composition of metabolites, so as to maintain the health of the body. In this paper, the effects of chemical composition and structural characterization of brown alga polysaccharides on intestinal microecology and mechanism of action were reviewed. Some suggestions for future development were put forward, such as improving the yield and purity of fucoidan, and further research on the structure-activity relationship and mechanism of fucoidan to provide reference for the development and application of brown alga polysaccharides.
Key words: intestinal microecology; intestinal barrier; intestinal flora; immune; brown alga polysaccharides


 A K,et al.Fucoidan structure and activity in relation to anti-cancer mechanisms[J].Marine Drugs,2019,17(1):32.
A K,et al.Fucoidan structure and activity in relation to anti-cancer mechanisms[J].Marine Drugs,2019,17(1):32.