罗非鱼(Oreochromis spp.),又称非洲鲫,是中国主养特色淡水鱼品种之一[1]。近年来,随着养殖规模的扩大,养殖环境的不稳定导致养殖罗非鱼深受无乳链球菌(Streptococcus agalactiae)的侵害[2]。一般来说,无乳链球菌在宿主体内主要以血液为载体,侵染机体各组织和器官[3]。无乳链球菌在血液中主要以三种形式存在:一是不黏附于任何结构,在血液中呈自由分散状态;二是被吞噬细胞和少量红细胞吞噬,躲避免疫识别;三是黏附于宿主血管内壁[4]。无乳链球菌穿透宿主细胞屏障到达血液,会触发机体清除病原体的免疫反应,但无乳链球菌也可以进入吞噬细胞和红细胞,并通过血液循环到达中枢神经系统,导致罗非鱼出现脑膜炎等症状甚至死亡[2,4]。因此,了解罗非鱼和无乳链球菌互作的血液免疫和侵染机制,对链球菌病的防治具有重要意义。
罗非鱼感染无乳链球菌后,血液生理指标如红细胞数、粒细胞数、白细胞数和血细胞比容等[5]降低,生化指标如白蛋白、球蛋白和溶菌酶活性等[5-6]升高,其生理生化指标变化与机体损伤、炎症及免疫反应密切相关。进一步研究无乳链球菌对罗非鱼的致病因素发现,该菌感染与血液多种通路如补体和凝血级联信号通路、血小板活化等[5]密切相关,其中,多种基因如TCR β[7]、CD2BP2[8]、CD59[9]、OnTF[10]、CD2[8]、MCP-8[11]、IL-37[12]等也已被挖掘,但无乳链球菌侵染罗非鱼血液相关的分子机制仍不明确。
了解宿主-病原体相互作用的方法之一可通过研究蛋白差异表达来剖析其分子机制。蛋白组学是一门新兴的生物研究技术,2019年,Wang等[13]使用蛋白组学技术,在患有红细胞增多症疾病(high-altitude polycythemia,HAPC)人类血浆中成功筛选出用于诊断和治疗的有效分子标记C4A、C6、CALR、MASP1和CNDP1。2020年,Fratini等[14]通过血浆外泌体的蛋白质组学分析,获得人囊性包虫病患者体内活动性和非活动性感染中潜在的分子标记物Src家族激酶Src和Lyn等;Zhou等[5]通过蛋白组学技术从无乳链球菌减毒毒株疫苗YM001与罗非鱼互作中筛选出菌体降低毒力的关键蛋白CSPA。同时,血浆蛋白质组学也被用于斑马贻贝(Dreissena polymorpha)抵抗小隐孢子虫感染免疫互作的分子机制研究[15]。因此,蛋白质组学技术可用于与病原感染过程中相关的潜在靶点挖掘和相关分子机制的研究。
罗非鱼感染无乳链球菌产生病理损伤的同时,会触发自身免疫保护机制。研究表明,罗非鱼脑对无乳链球菌入侵的免疫反应在6 h时已被激活,且此时与炎症和免疫相关的神经肽前体基因表达水平已发生显著变化[16]。此外,感染无乳链球菌6 h后,罗非鱼脾脏中与免疫细胞功能相关的MicroRNA的表达水平也发生了显著变化[17]。本研究中应用TMT标记定量蛋白组学技术研究了罗非鱼感染无乳链球菌6 h后,其血浆蛋白的差异表达及其相关的信号通路,以探讨血液在罗非鱼感染无乳链球菌过程中发挥的作用,以期为进一步研究罗非鱼感染无乳链球菌的分子机制提供基础资料。
1 材料与方法
1.1 材料
吉富尼罗罗非鱼(Oreochromis niloticus GIFT)来源于合作企业广东罗非鱼良种场,体质量为(150±10)g,试验在中国水产科学研究院珠江水产研究所养殖基地进行,86尾罗非鱼随机分成两组,在同种规格的养殖箱(0.8 m×1.0 m×2.5 m)暂养,期间每天投饵两次,日投饵量为体质量的3%,水温为(31±0.5)℃,按照常规饲养管理。
仪器设备:梅里埃浊度仪(99234)、高效液相色谱系统(RIGOL L-3000)、电热恒温水浴锅(XMTD-7000)、Eppendorf离心机(5430R)、酶标仪(DR200B)。
试剂:BCA蛋白定量试剂盒(Thermo)、ProteoMinerTM蛋白富集试剂盒(Bio-Rad)、胰蛋白酶(Promega)、二硫苏糖醇DTT(Amresco)、乙腈(J.T.Baker)、碘乙酰胺IAM(Amresco)、甲酸(Sigma-Aldrich)、碳酸氢铵(Sigma-Aldrich)、氨水(Wako Pure Chemical Industries Ltd)、TMT16plexTM Isobaric Label Reagent Set(Thermo)、C18色谱柱(Waters)。
1.2 方法
1.2.1 无乳链球菌毒株培养 将无乳链球菌菌株WC1535(本实验室保存)接种于血平板,37 ℃下培养24 h,BHI液体培养基扩培后,离心获取菌体,PBS稀释后,使用梅里埃浊度仪将菌液浓度调整至半致死剂量(LD50=5×107 CFU/mL,LD50通过预试验获取)。
1.2.2 罗非鱼攻毒试验 攻毒试验前禁食24 h,攻毒试验分为试验组和对照组,每组43尾,试验组每尾鱼腹腔内注射浓度为5×107 CFU/mL的WC1535菌液100 μL,对照组每尾鱼腹腔内注射无菌PBS溶液100 μL。罗非鱼感染无乳链球菌6 h后,分别随机采集两组各12尾鱼的血液样品。用无菌含EDTA抗凝剂的注射器采集全血,采集后迅速离心(1 600×g,15 min,4 ℃),收集离心后的上清液即为血浆,试验组标记为TP,对照组标记为TPC,储存于液氮中,每组随机设置3个生物学重复用于蛋白组学测定。
1.2.3 血浆蛋白提取及定量 血浆用裂解缓冲液稀释后混匀,冰上静置10 min,离心(12 000×g,20 min,4 ℃)取上清,上清为血浆总蛋白。使用ProteoMinerTM蛋白富集试剂盒(Bio-Rad)去除总蛋白中高丰度蛋白质,然后采用Bradford法[18]测定每个样品的蛋白浓度。由苏州帕诺米克生物医药科技有限公司进行蛋白组学分析。
1.2.4 蛋白酶解和脱盐 蛋白样品中加入DTT,37 ℃下孵育1 h,然后加入IAM室温避光孵育45 min。用碳酸氢铵稀释样品后加入胰酶,37 ℃下孵育过夜,最后加入甲酸终止酶切。使用C18脱盐柱对样本进行脱盐,收集流穿液。
1.2.5 TMT标记和LC-MS/MS质谱分析 样品中加入TMT混合标记后,冻干。混合标记后的样本用体积分数为0.1%甲酸溶解,使用高效液相进行肽段分级处理,收集洗脱物冷冻干燥。使用ORBITRAP ECLIPSE 质谱仪进行质谱检测,生成质谱检测原始数据。
1.2.6 差异蛋白Western blot验证 为验证TMT蛋白组学结果,选取目标差异表达蛋白A0A669EZW6(IGK),克隆cds片段,通过体外表达获取蛋白抗原。利用体外表达的蛋白抗原免疫新西兰大白兔,获取蛋白IGK多克隆抗体(多克隆抗体制备由武汉金开瑞生物工程有限公司完成),利用抗体对感染无乳链球菌的罗非鱼血浆进行Western blot检测验证。蛋白样品使用BCA蛋白定量试剂盒测定浓度后,进行SDS-PAGE电泳并转膜,5%脱脂牛奶封闭1 h,洗涤后加入稀释的一抗(5 μg/mL),37 ℃下孵育2 h,洗涤后加入稀释的二抗(1∶5 000)孵育1 h,洗涤后进行显影。IGK的相对表达量为IGK灰度值与GAPDH灰度值的比值,采用Image J软件检测每个条带的灰度值,对数据进行T检验分析,采用Excel 2010软件绘制柱形图。
1.3 数据处理
质谱原始数据导入Proteome Discoverer 2.4.1.15软件,进行数据库检索(Uniprot_Oreochromis_niloticus_76006_20221117_2022)、肽段与蛋白鉴定、蛋白定量。通过GraphPad Prism v8.0.2.263软件进行数据统计T检验分析,计算组间蛋白差异倍数(fold change,FC)和显著性P值。以FC>1.2或FC<0.83且P<0.05为标准筛选显著差异表达蛋白(significantly differentially expressed proteins,SDEPs)。将显著差异蛋白氨基酸序列在NR数据库中进行序列比对,获得蛋白注释信息。最后在BioDeep平台(https://www.biodeep.cn)上对数据进行GO和KEGG通路富集分析。
2 结果与分析
2.1 蛋白定量数据质量评价
血浆蛋白经胰蛋白酶消化和TMT标记后,使用液相色谱技术进行质谱检测,检测结束后,所获得的数据进行数据库检索,从图1(a)、(b)可见,蛋白定量值采用中位数法进行归一化处理,样本所有蛋白矫正前后定量值水平保持一致,即中位线处于同一水平线,说明矫正结果较好;图1(c)显示每个样本均定量出751个蛋白;图1(d)显示两组样本之间所有定量蛋白的偏最小二乘法判别分析,试验组和对照组两组样本相距距离较远,明显分离,表明两组样本存在差异;以上结果表明,本研究中得到的数据具有良好的质量,这为后续分析提供了可靠的依据。
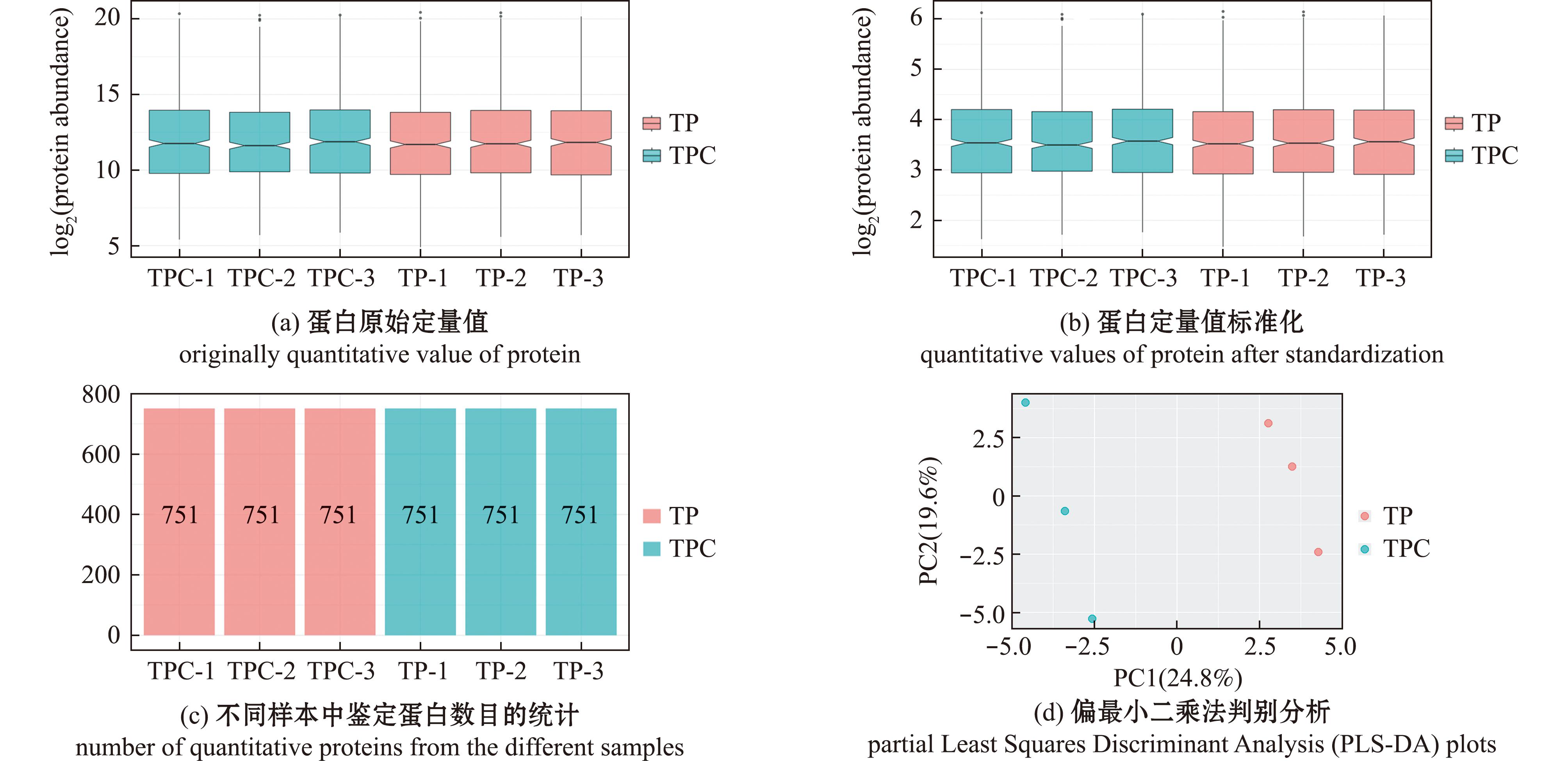
TP-1~3为试验组;TPC-1~3为对照组。TP-1-3 represent the experimental groups;TPC-1-3 represent the control group.
图1 不同样品血浆总蛋白的质量评价分析
Fig.1 Quality evaluation of quantitative proteins from different plasma samples
2.2 差异蛋白筛选
根据条件筛选发现,试验组与对照组相比,共获得34个SDEPs,其中,9个上调SDEPs(A2M、CD163、CD86、CFHR5、CD162等),25个下调SDEPs(IGK、IGH、IGV、egfr等),并对SDEPs进行NR注释(表1)和火山图绘制(图2)。通过对SDEPs进行聚类分析(图3),组内平行样本间SDEPs表达模式基本一致,而试验组与对照组相比,SDEPs表达有显著性差异。
表1 显著差异蛋白NR注释
Tab.1 NR annotation of significantly differentially expressed proteins
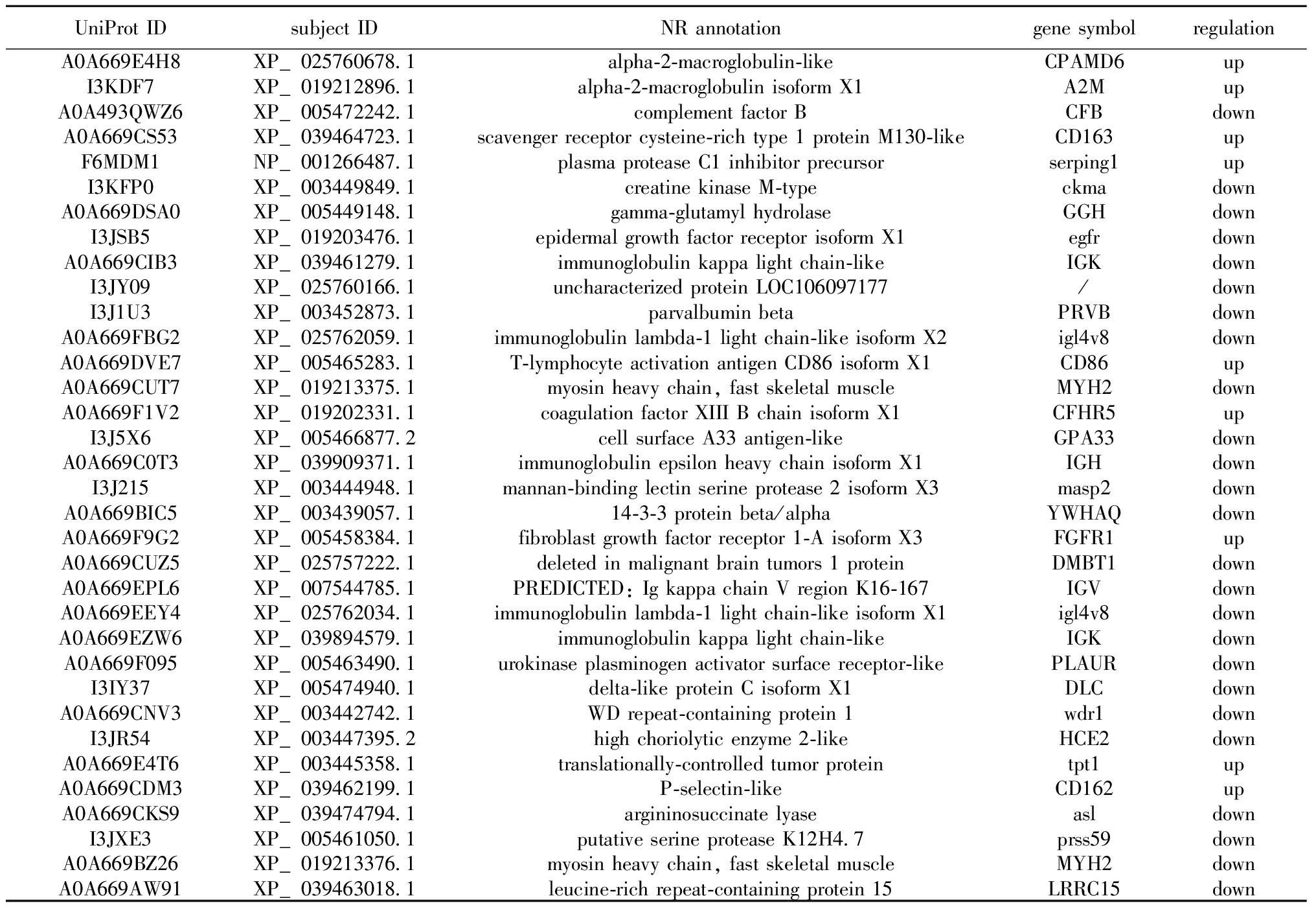
UniProt IDsubject IDNR annotationgene symbolregulationA0A669E4H8XP_025760678.1alpha-2-macroglobulin-likeCPAMD6upI3KDF7XP_019212896.1alpha-2-macroglobulin isoform X1A2MupA0A493QWZ6XP_005472242.1complement factor BCFBdownA0A669CS53XP_039464723.1scavenger receptor cysteine-rich type 1 protein M130-likeCD163upF6MDM1NP_001266487.1plasma protease C1 inhibitor precursorserping1upI3KFP0XP_003449849.1creatine kinase M-typeckmadownA0A669DSA0XP_005449148.1gamma-glutamyl hydrolaseGGHdownI3JSB5XP_019203476.1epidermal growth factor receptor isoform X1egfrdownA0A669CIB3XP_039461279.1immunoglobulin kappa light chain-likeIGKdownI3JY09XP_025760166.1uncharacterized protein LOC106097177/downI3J1U3XP_003452873.1parvalbumin betaPRVBdownA0A669FBG2XP_025762059.1immunoglobulin lambda-1 light chain-like isoform X2igl4v8downA0A669DVE7XP_005465283.1T-lymphocyte activation antigen CD86 isoform X1CD86upA0A669CUT7XP_019213375.1myosin heavy chain, fast skeletal muscleMYH2downA0A669F1V2XP_019202331.1coagulation factor XIII B chain isoform X1CFHR5upI3J5X6XP_005466877.2cell surface A33 antigen-likeGPA33downA0A669C0T3XP_039909371.1immunoglobulin epsilon heavy chain isoform X1IGHdownI3J215XP_003444948.1mannan-binding lectin serine protease 2 isoform X3masp2downA0A669BIC5XP_003439057.114-3-3 protein beta/alphaYWHAQdownA0A669F9G2XP_005458384.1fibroblast growth factor receptor 1-A isoform X3FGFR1upA0A669CUZ5XP_025757222.1deleted in malignant brain tumors 1 proteinDMBT1downA0A669EPL6XP_007544785.1PREDICTED: Ig kappa chain V region K16-167IGVdownA0A669EEY4XP_025762034.1immunoglobulin lambda-1 light chain-like isoform X1igl4v8downA0A669EZW6XP_039894579.1immunoglobulin kappa light chain-likeIGKdownA0A669F095XP_005463490.1urokinase plasminogen activator surface receptor-likePLAURdownI3IY37XP_005474940.1delta-like protein C isoform X1DLCdownA0A669CNV3XP_003442742.1WD repeat-containing protein 1wdr1downI3JR54XP_003447395.2high choriolytic enzyme 2-likeHCE2downA0A669E4T6XP_003445358.1translationally-controlled tumor proteintpt1upA0A669CDM3XP_039462199.1P-selectin-likeCD162upA0A669CKS9XP_039474794.1argininosuccinate lyaseasldownI3JXE3XP_005461050.1putative serine protease K12H4.7prss59downA0A669BZ26XP_019213376.1myosin heavy chain, fast skeletal muscleMYH2downA0A669AW91XP_039463018.1leucine-rich repeat-containing protein 15LRRC15down
注:UniProt ID为差异蛋白的氨基酸序列的ID;subject ID为NR数据库比对序列的ID;NR annotation为NR数据库比对序列的注释;gene symbol为基因符号;regulation为差异蛋白的表达情况。
Note:UniProt ID is the ID of the amino acid sequence of the SDEPs;subject ID is ID of the sequence of NR database comparison;NR annotation is the annotation of the sequence of the NR database comparison;gene symbol is the name of the gene;regulation is the expression of SDEPs.
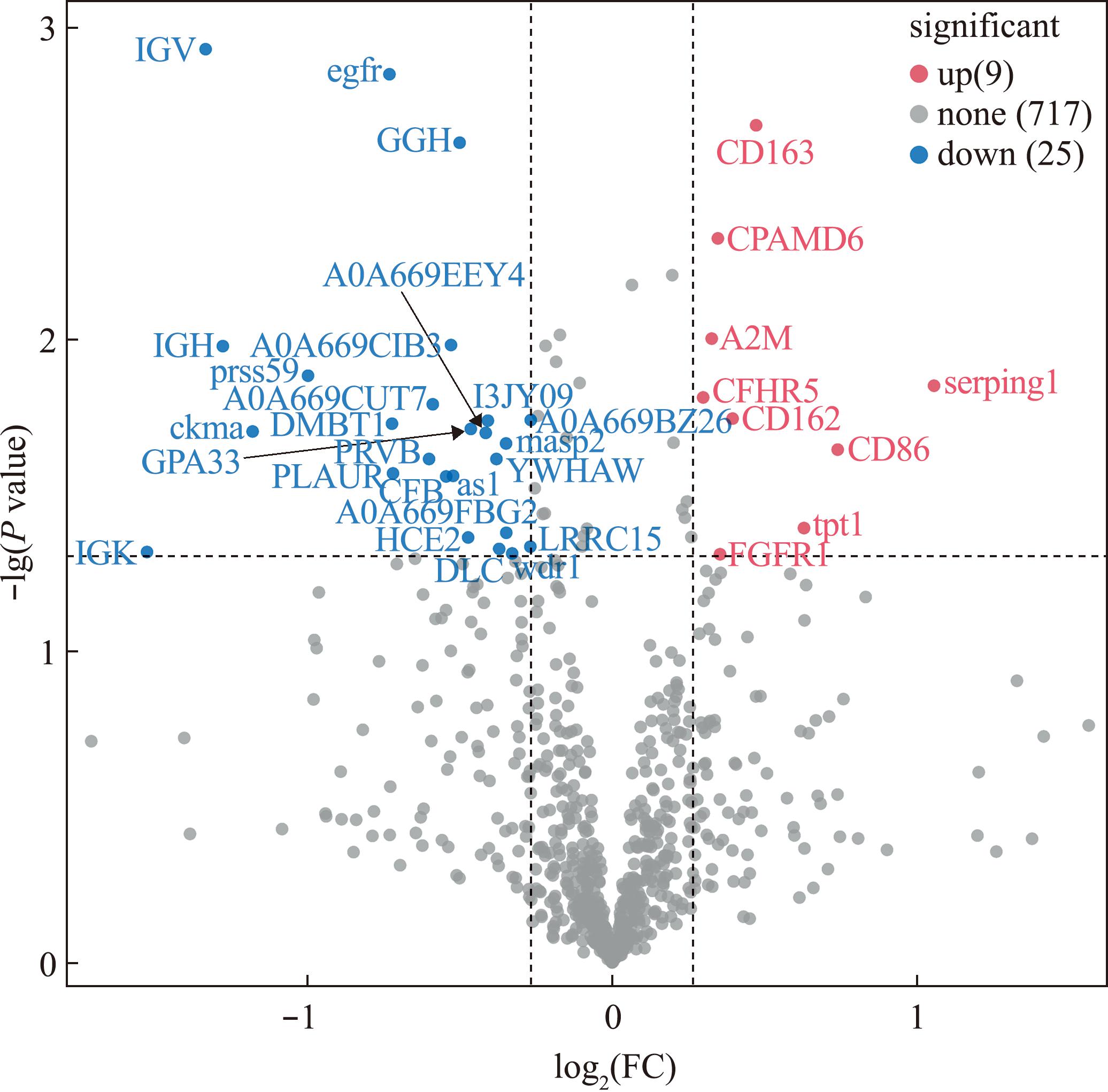
图2 差异蛋白火山图
Fig.2 Volcano plot of significantly differentially expressed proteins(SDEPs)
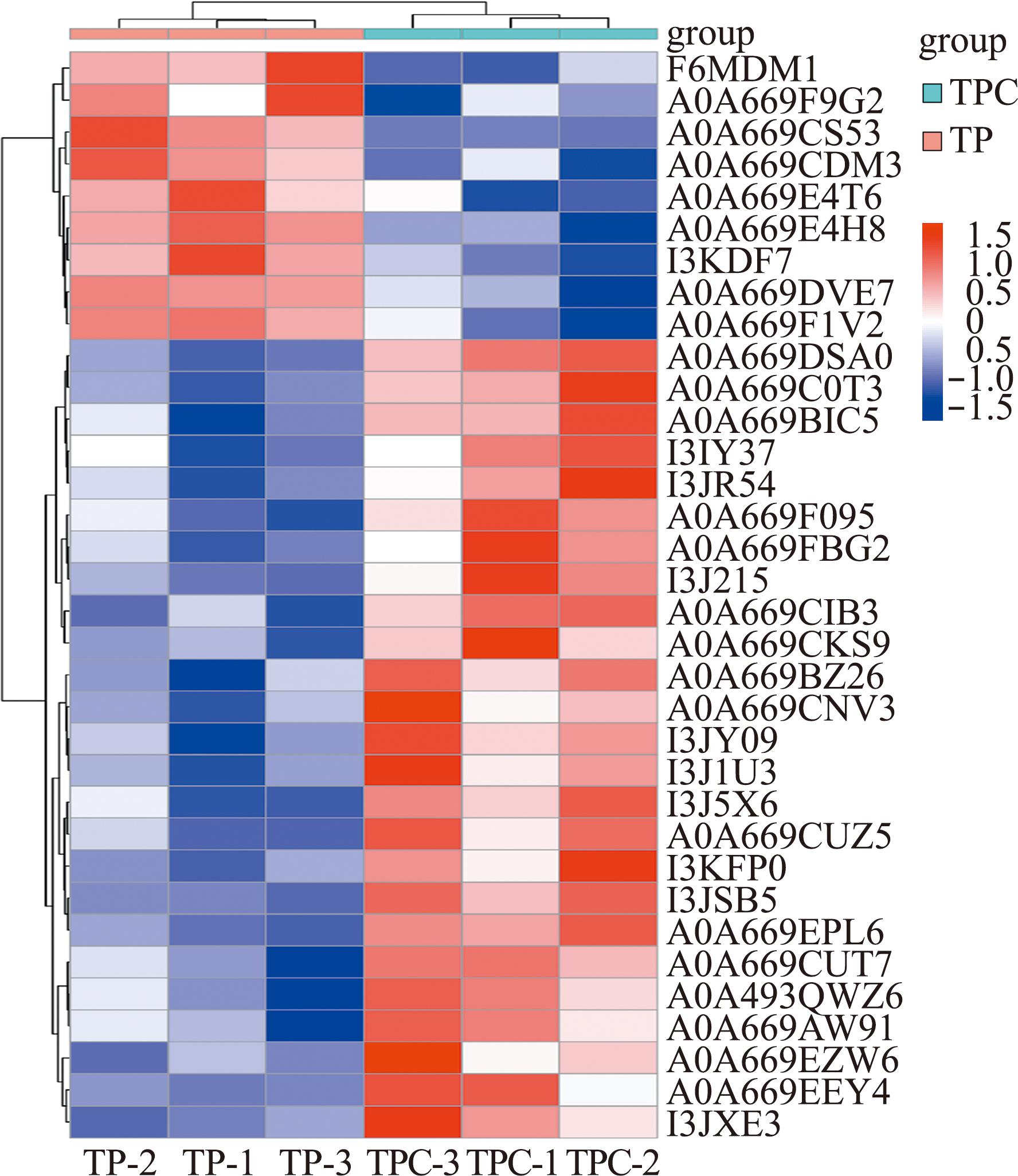
图3 差异蛋白聚类热图
Fig.3 Heat map of significantly differentially expressed proteins
2.3 差异表达蛋白GO富集分析
对SDEPs进行GO富集分析(图4),结果显示,SDEPs主要参与的生物学过程为蛋白质水解(proteolysis)、解剖结构的发育(anatomical structure development),具有三磷酸腺苷结合(ATP binding)、细胞骨架运动活性(cytoskeletal motor activity)、丝氨酸型内肽酶抑制剂活性(serine-type endopeptidase inhibitor activity)、跨膜受体蛋白酪氨酸激酶活性(transmembrane receptor protein tyrosine kinase activity)、肌动蛋白丝结合(actin filament binding)重要分子功能,并与整合膜(integral component of membrane)、肌球蛋白复合物(myosin complex)细胞组分密切相关。
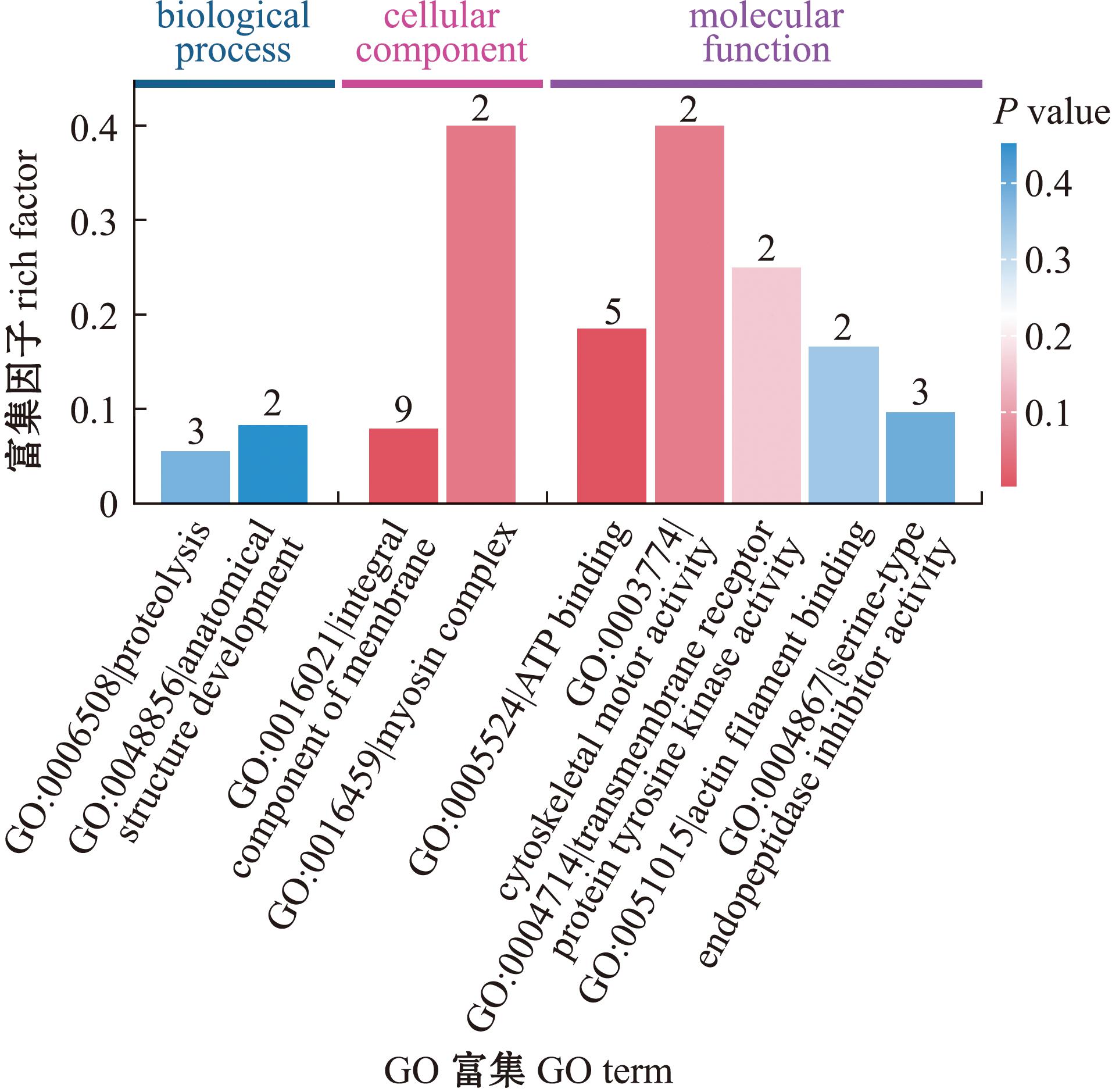
图4 差异蛋白GO富集分析
Fig.4 GO enrichment analysis of significantly differentially expressed proteins
2.4 差异表达蛋白KEGG富集分析
KEGG富集分析结果显示,SDEPs显著富集于肌动蛋白细胞骨架调节(regulation of actin cytoskeleton)、血管平滑肌收缩(vascular smooth muscle contraction)、内分泌抵抗(endocrine resistance)、MAPK信号通路(MAPK signaling pathway)、癌症中心碳代谢(central carbon metabolism in cancer)、癌症相关蛋白聚糖(proteoglycans in cancer)、乳腺癌(breast cancer)、紧密连接(tight junction)和Ras信号通路(Ras signaling pathway)等通路(图5)。其中,肌动蛋白细胞骨架调节、乳腺癌和癌症相关蛋白聚糖通路是富集蛋白最多的信号通路。
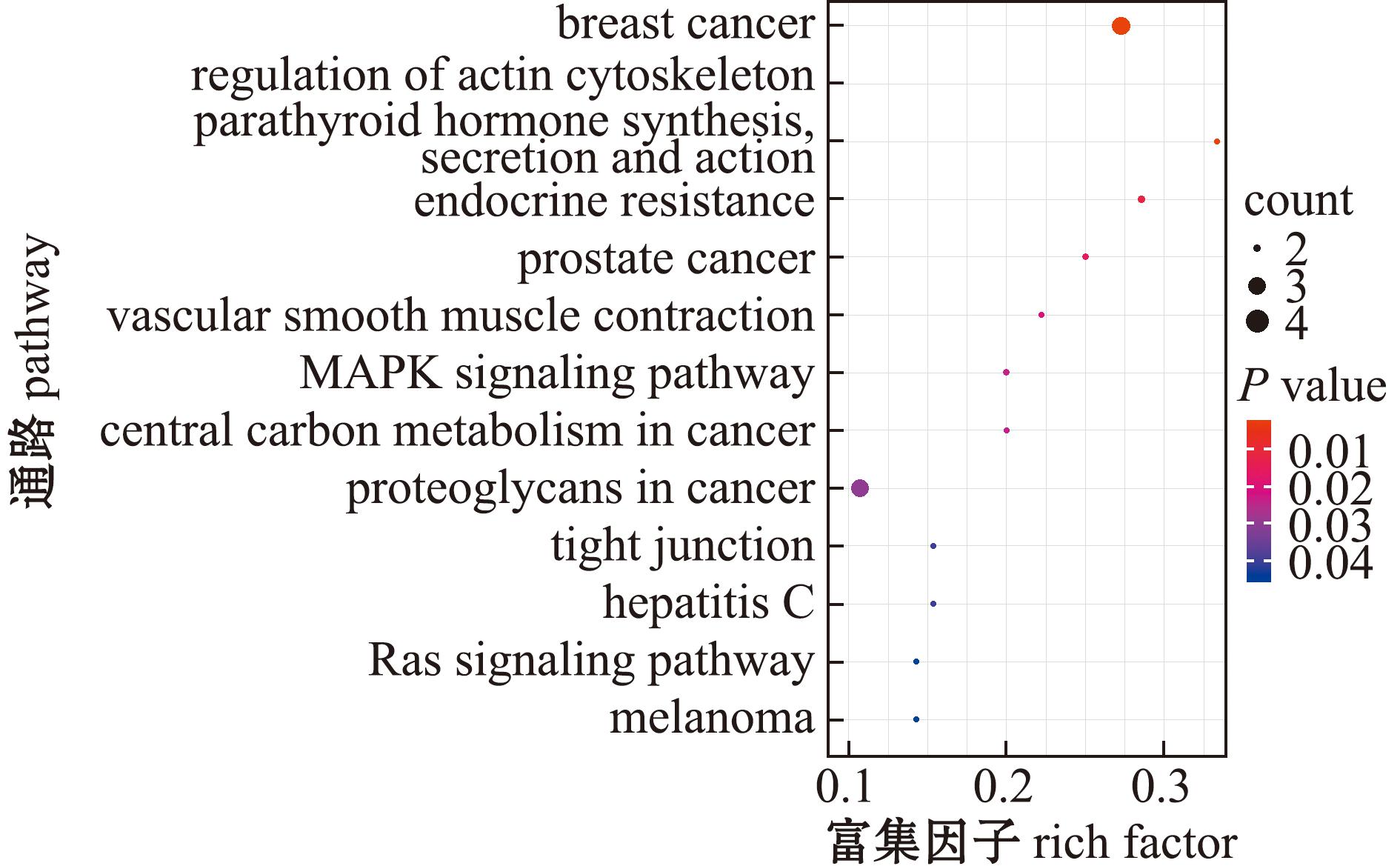
图5 差异蛋白KEGG通路富集分析
Fig.5 KEGG pathway enrichment analysis of significantly differentially expressed proteins
通过构建通路与SDEPs的相互作用关系图发现,差异蛋白PLAUR、DLC、egfr、FGFR1、MYH2、YWHAQ富集于多条通路,其中,蛋白egfr和FGFR1连接多种通路如肌动蛋白细胞骨架调节、内分泌抵抗、MAPK信号通路、癌症中心碳代谢、癌症相关蛋白聚糖、紧密连接和Ras信号通路等,是以上通路网络的中心蛋白(图6)。
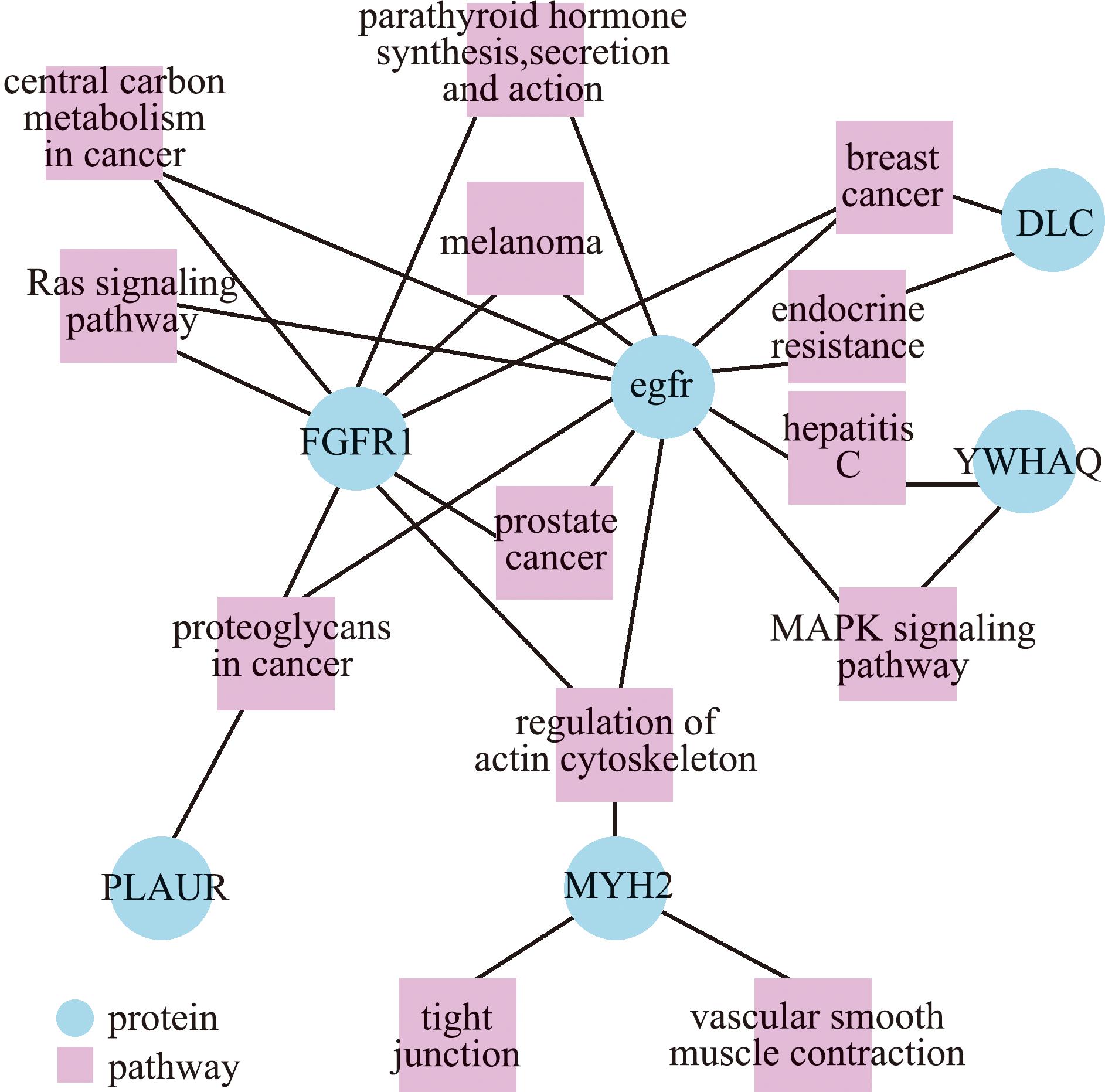
图6 KEGG富集通路与差异蛋白相互关系
Fig.6 Correlation analysis between KEGG pathway and significantly differentially expressed proteins
2.5 差异蛋白Western blot验证
为了验证所筛选差异蛋白质的表达变化,选取蛋白IGK进行多克隆抗体的制备并利用Western blot进行验证。结果表明,与对照组相比,试验组血浆IGK蛋白丰度显著降低(P<0.05),其蛋白表达丰度变化与蛋白质组学分析结果一致(图7)。

*代表P<0.05。
* means P<0.05.
图7 差异蛋白Western blot验证
Fig.7 Western blot verification of differential proteins
3 讨论
3.1 罗非鱼感染无乳链球菌后血浆蛋白组学差异蛋白表达分析
罗非鱼对无乳链球菌高度易感,可引起暴发性死亡,严重影响了罗非鱼产业的发展。如何有效控制无乳链球菌病引起的危害对罗非鱼产业健康发展至关重要。目前,国内外学者对无乳链球菌的致病机理、免疫逃逸机制、疫苗开发等均开展了大量研究,但罗非鱼血液在无乳链球菌感染过程中的作用机制研究相对较少。本研究中通过TMT定量蛋白组学技术探讨罗非鱼感染无乳链球菌前后血浆蛋白的差异表达情况,以进一步提高人们对无乳链球菌感染罗非鱼过程中血液作用的认识。罗非鱼感染无乳链球菌6 h后,血液血浆的蛋白表达发生了显著变化,本研究中共鉴定出751个定量差异蛋白,筛选出34个SDEPs,其中25个SDEPs在试验组中显著下调,9个SDEPs在试验组中显著上调。在蛋白质差异表达的聚类分析中,组内平行样品表达模式相似性较高,而试验组和对照组样品表达差异显著,说明两组样品重复性良好,且两组样品中蛋白表达水平的变化是有本质区别的。进一步通过GO和KEGG分析发现,这些SDEPs主要参与ATP能量代谢、细胞运动和免疫调节等多个生物学过程。
3.2 罗非鱼感染无乳链球菌后血浆显著差异蛋白能量代谢相关分析
本研究中,通过对罗非鱼感染无乳链球菌后血浆显著差异蛋白进行GO和KEGG富集分析,发现血浆蛋白ckma、egfr和FGFR1具有ATP结合的分子功能,其中egfr、FGFR1显著富集于癌症中心碳代谢通路。中心碳代谢是机体能量的主要来源,而ATP代谢与机体炎症反应和免疫调控密切相关。在炎症反应方面,细胞外ATP是损伤相关模式分子,可通过与质膜上的嘌呤能受体结合,诱导炎症反应,激活宿主先天免疫[19]。研究发现,在炎症的早期阶段,高水平的细胞外ATP具有促炎作用,可刺激免疫细胞表达促炎细胞因子,产生iNOS活性和炎症介质(ROS和NO) [19]。为了避免ATP诱导的过度炎症病理效应,磷酸酶可以水解ATP,从而增加腺苷水平。腺苷不仅诱导单核细胞释放抗炎细胞因子IL-10,还诱导单核细胞产生血管内皮生长因子来促进血管生成和伤口修复,从而参与炎症的消退[20],而低水平ATP则被证明具有与腺苷类似的抗炎作用[21]。在特异性免疫方面,高水平ATP抑制调节性T细胞功能,并诱导调节性T细胞的膜孔形成和凋亡,而低水平ATP则刺激调节性T细胞增殖、细胞因子释放、黏附分子表达和黏附[22-23]。在本研究中,与ATP代谢相关的ckma、egfr、MYH2、FGFR1等差异蛋白表达的变化,可能是通过ATP代谢水平的变化来介导罗非鱼感染无乳链球菌后的炎症及免疫反应的重要依据。
此外,ckma在维持细胞能量的稳态中发挥重要作用。研究发现,银鲶(Rhamdia quelen)在受到无乳链球菌刺激后,大脑中ckma活性下降[24],引起与ATP代谢水平相关的能量失衡[25],引发脑膜炎损伤,导致中枢神经系统发病,因此,本研究中ckma蛋白水平显著下调,猜测可能与无乳链球菌引起罗非鱼脑膜炎密切相关。
3.3 罗非鱼感染无乳链球菌后血浆显著差异蛋白细胞运动相关分析
本研究中,多个与细胞运动过程相关的SDEPs显著富集于肌动蛋白细胞骨架调节、癌症相关蛋白聚糖、血管平滑肌收缩等信号通路,通过GO富集分析发现,这些SDEPs主要具有跨膜受体蛋白酪氨酸激酶活性、细胞骨架运动活性、肌动蛋白丝结合等细胞运动相关分子功能。其中,肌动蛋白细胞骨架调节和癌症相关蛋白聚糖通路富集的差异蛋白最多。肌动蛋白参与内吞、吞噬、细胞分裂等细胞运动过程,对维持细胞的形态和功能具有重要作用[26]。病原菌在入侵机体过程中,肌动蛋白细胞骨架是细菌的首选靶点[27]。细菌通过改变或破坏宿主细胞的屏障侵入细胞,以促进自身在细胞间扩散,并通过调节肌动蛋白细胞骨架动力学,抑制肌动蛋白重排来阻止细胞吞噬[26],这可能是罗非鱼感染无乳链球菌后,其血浆肌动蛋白细胞骨架调节相关蛋白表达发生显著变化的主要原因。研究发现,沙门氏菌(Salmonella enterica)可在巨噬细胞中存活和复制,会同时激活宿主调节细胞骨架动力学,并通过减少细胞运动、抑制细菌入侵、诱导抗菌分子产生等方式有效清除细菌,从而减少细胞内细菌负荷量[28]。因此,宿主肌动蛋白细胞骨架的改变可能在细胞免疫防御中也发挥着重要作用。
癌症相关蛋白聚糖通路在炎症和免疫反应等过程中介导细胞信号传递,蛋白聚糖是广泛存在于各种组织的糖基化修饰蛋白,与生长因子、黏附分子等相互作用,参与细胞黏附、迁移、增殖及分化,并且参与组织修复、血管生成等基质微环境的重塑和维持组织稳态[29]。其次,病原菌可通过蛋白聚糖黏附和入侵宿主细胞,调控宿主的炎症反应,诱导细胞表面蛋白聚糖脱落,从而逃避或抑制宿主的免疫防御[30]。因此,本研究中罗非鱼感染无乳链球菌血浆蛋白聚糖相关蛋白的差异表达可能与机体炎症反应和免疫防御有关。
3.4 罗非鱼感染无乳链球菌后血浆显著差异蛋白免疫调节分析
本研究中罗非鱼感染无乳链球菌6 h后,血浆CD家族蛋白(CD86、CD162和CD163)显著上调,IG类蛋白(IGK、IGH、IGV和igl4v8)显著下调,可能与罗非鱼免疫细胞抗原识别和炎症反应密切相关。CD家族蛋白是白细胞及其他免疫细胞的细胞表面分子,通常用作细胞标记物,对免疫细胞间的信息传导和微环境感知起着关键作用。CD86参与早期免疫反应,在幼稚T细胞和记忆T细胞的活化中发挥重要作用[31];CD162是一种黏附分子,在急性炎症反应中介导中性粒细胞募集[32];CD163充当先天免疫传感器和局部炎症的诱导剂,可作为巨噬细胞和细菌黏附的结合位点,促进单核细胞炎性细胞因子产生[33]。IG类蛋白则是免疫系统中的关键效应蛋白,如IGK和IGH是B细胞膜表面受体免疫球蛋白的组成成分,B细胞受体可以识别抗原并与之结合,介导机体免疫反应[34]。同时,IG类蛋白基因可以重排,使得B细胞免疫球蛋白具有多样性,可以识别数以万计的不同抗原。
此外,本研究中,罗非鱼egfr、GPA33、masp2、YWHAQ和DMBT1等均发生了显著变化,其中,egfr(酪氨酸激酶)是连接多条富集通路的中心蛋白。酪氨酸激酶是质膜相关蛋白,充当信号分子控制免疫细胞的生长、增殖和调控细胞周期,在病原体识别、巨噬细胞介导的炎症反应和淋巴细胞免疫应答等方面发挥着重要作用[35]。病原体可以通过激活宿主细胞表面的酪氨酸激酶或其受体,调控宿主的肌动蛋白骨架动力学,从而入侵宿主并传播致病因子,改变质膜结构,激活调节宿主免疫防御系统的信号通路[36]。研究表明,酪氨酸激酶抑制剂可以促进巨噬细胞溶酶体酸化,从而有效地抑制致病菌的生长[37]。因此,egfr可能参与罗非鱼巨噬细胞介导的炎症反应,且在白细胞免疫应答中发挥着重要免疫防御作用[38]。
4 结论
1)通过分析罗非鱼感染无乳链球菌6 h后血浆蛋白的差异表达,发现无乳链球菌感染影响罗非鱼血浆蛋白表达水平,SDEPs主要富集在肌动蛋白细胞骨架调节、癌症相关蛋白聚糖等多个通路。
2)无乳链球菌感染对罗非鱼的能量代谢、细胞运动及免疫反应等生物学过程具有显著影响,本研究结果为进一步解析罗非鱼感染无乳链球菌相关的分子机制提供了数据参考。
[1] 刘志刚,高风英,佟延南,等.尼罗罗非鱼生长性状相关微卫星标记的筛选与验证[J].大连海洋大学学报,2023,38(6):925-934.
LIU Z G,GAO F Y,TONG Y N,et al.Screening and verification of microsatellite markers associated with growth traits in Nile tilapia (Oreochromis niloticus)[J].Journal of Dalian Ocean University,2023,38(6):925-934.(in Chinese)
[2] LIU Y,LI L P,HUANG T,et al.The interaction between phagocytes and Streptococcus agalactiae (GBS) mediated by the activated complement system is the key to GBS inducing acute bacterial meningitis of tilapia[J].Animals:an Open Access Journal from MDPI,2019,9(10):818.
[3] 苏友禄,刘婵,邓益琴,等.罗非鱼无乳链球菌病的研究进展[J].大连海洋大学学报,2019,34(5):757-766.
SU Y L,LIU C,DENG Y Q,et al.Research on Streptococcus agalactiae disease in tilapia:a review[J].Journal of Dalian Ocean University,2019,34(5):757-766.(in Chinese)
[4] CAO J,LIU Z,ZHANG D,et al.Distribution and localization of Streptococcus agalactiae in different tissues of artificially infected tilapia (Oreochromis niloticus)[J]. Aquaculture, 2022, 546: 737370.
[5] ZHOU Y,LIU Y,LUO Y J,et al.Large-scale profiling of the proteome and dual transcriptome in Nile tilapia (Oreochromis niloticus) challenged with low- and high-virulence strains of Streptococcus agalactiae[J].Fish &Shellfish Immunology,2020,100:386-396.
[6] YI M M,WANG M,LI Z H,et al.An investigation into the effects of Streptococcus agalactiae on the 5-HT system and the behavior of GIFT tilapia (Oreochromis niloticus)[J].Aquaculture Reports,2019,15:100232.
[7] NITHIKULWORAWONG N,YAKUPITIYAGE A,RAKSHIT S K,et al.Molecular characterization and increased expression of the Nile tilapia,Oreochromis niloticus (L.),T-cell receptor beta chain in response to Streptococcus agalactiae infection[J].Journal of Fish Diseases,2012,35(5):343-358.
[8] GAN Z,WANG B,ZHOU W,et al.Molecular characterization and expression of ZAP-70 in Nile tilapia (Oreochromis niloticus) in response to Streptococcus agalactiae stimulus[J].Genes &Genomics,2016,38(4):321-331.
[9] GAN Z,WANG B,ZHOU W,et al.Molecular and functional characterization of CD59 from Nile tilapia (Oreochromis niloticus) involved in the immune response to Streptococcus agalactiae[J].Fish &Shellfish Immunology,2015,44(1):50-59.
[10] POOCHAI W,CHOOWONGKOMON K,SRISAPOOME P,et al.Characterization and expression analysis of the transferrin gene in Nile tilapia (Oreochromis niloticus) and its upregulation in response to Streptococcus agalactiae infection[J].Fish Physiology and Biochemistry,2014,40(5):1473-1485.
[11] FU G H,WAN Z Y,XIA J H,et al.The MCP-8 gene and its possible association with resistance to Streptococcus agalactiae in tilapia[J].Fish &Shellfish Immunology,2014,40(1):331-336.
[12] DE SOUSA E L,ASSANE I M,SANTOS-FILHO N A,et al.Haematological, biochemical and immunological biomarkers, antibacterial activity, and survival in Nile tilapia Oreochromis niloticus after treatment using antimicrobial peptide LL-37 against Streptococcus agalactiae[J].Aquaculture,2021,533(1):736181.
[13] WANG Z K,LIU F J,YE S L,et al.Plasma proteome profiling of high-altitude polycythemia using TMT-based quantitative proteomics approach[J].Journal of Proteomics,2019,194:60-69.
[14] FRATINI F,TAMAROZZI F,MACCHIA G,et al.Proteomic analysis of plasma exosomes from cystic echinococcosis patients provides in vivo support for distinct immune response profiles in active vs inactive infection and suggests potential biomarkers[J].PLoS Neglected Tropical Diseases,2020,14(10):e0008586.
[15] LEPR TRE M,ALMUNIA C,ARMENGAUD J,et al.Identification of immune-related proteins of Dreissena polymorpha hemocytes and plasma involved in host-microbe interactions by differential proteomics[J].Scientific Reports,2020,10(1):6226.
TRE M,ALMUNIA C,ARMENGAUD J,et al.Identification of immune-related proteins of Dreissena polymorpha hemocytes and plasma involved in host-microbe interactions by differential proteomics[J].Scientific Reports,2020,10(1):6226.
[16] LI Q,LIU R N,MA R R,et al.Brain transcriptome response to Streptococcus agalactiae infection and the heterogeneous regulation of neuropeptides on immune response in tilapia,Oreochromis niloticus[J].Aquaculture,2022,555:738222.
[17] WANG B,GAN Z,WANG Z L,et al.Integrated analysis neurimmiRs of tilapia (Oreochromis niloticus) involved in immune response to Streptococcus agalactiae,a pathogen causing meningoencephalitis in teleosts[J].Fish &Shellfish Immunology,2017,61:44-60.
[18] BRADFORD M M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J].Analytical Biochemistry,1976,72:248-254.
[19] LI S,CHEN X L,LI J F,et al.Extracellular ATP is a potent signaling molecule in the activation of the Japanese flounder (Paralichthys olivaceus) innate immune responses[J].Innate Immunity,2020,26(5):413-423.
[20] Faas M, Sáez T, De Vos P. Extracellular ATP and adenosine: the Yin and Yang in immune responses?[J]. Molecular Aspects of Medicine, 2017, 55: 9-19.
[21] HILL L M, GAVALA M L, LENERTZ L Y, et al. Extracellular ATP may contribute to tissue repair by rapidly stimulating purinergic receptor X7-dependent vascular endothelial growth factor release from primary human monocytes[J]. The Journal of Immunology, 2010, 185(5): 3028-3034.
[22] BAO R, HOU J, LI Y,et al. Adenosine promotes Foxp3 expression in Treg cells in sepsis model by activating JNK/AP-1 pathway[J]. American Journal of Translational Research, 2016, 8(5): 2284.
[23] TRABANELLI S, OADLíKOVá D, GULINELLI S, et al. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation[J]. The Journal of Immunology, 2012, 189(3): 1303-1310.
[24] Baldissera M D, Souza C F, Parmeggiani B S,et al. Streptococcus agalactiae impairs cerebral bioenergetics in experimentally infected silver catfish[J]. Microbial Pathogenesis, 2017, 111: 28-32.
[25] BALDISSERA M D,SOUZA C F,SANTOS R C,et al.Streptococcus agalactiae alters cerebral enzymes of phosphoryl transfer network in experimentally infected silver catfish:impairment on brain energy homeostasis[J].Aquaculture,2018,489:105-109.
[26] KÜHN S,MANNHERZ H G.Actin:structure,function,dynamics,and interactions with bacterial toxins[J].Current Topics in Microbiology and Immunology,2017,399:1-34.
[27] LAMASON R L,WELCH M D.Actin-based motility and cell-to-cell spread of bacterial pathogens[J].Current Opinion in Microbiology,2017,35:48-57.
[28] MAN S M,EKPENYONG A,TOURLOMOUSIS P,et al.Actin polymerization as a key innate immune effector mechanism to control Salmonella infection[J].Proceedings of the National Academy of Sciences of the United States of America,2014,111(49):17588-17593.
[29] TUMOVA S,WOODS A,COUCHMAN J R.Heparan sulfate proteoglycans on the cell surface:versatile coordinators of cellular functions[J].The International Journal of Biochemistry &Cell Biology,2000,32(3):269-288.
[30] CHEN Y,GÖTTE M,LIU J,et al.Microbial subversion of heparan sulfate proteoglycans[J].Molecules and Cells,2008,26(5):415-426.
[31] JEANNIN P,MAGISTRELLI G,AUBRY J P,et al.Soluble CD86 is a costimulatory molecule for human T lymphocytes[J].Immunity,2000,13(3):303-312.
[32] ZHANG S E,SONG L,WANG Y Z,et al.Targeting CD162 protects against streptococcal M1 protein-evoked neutrophil recruitment and lung injury[J].American Journal of Physiology Lung Cellular and Molecular Physiology,2013,305(10):L756-L763.
[33] FABRIEK B O,VAN BRUGGEN R,DENG D M,et al.The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria[J].Blood,2009,113(4):887-892.
[34] SAYEGH C E,JHUNJHUNWALA S,RIBLET R,et al.Visualization of looping involving the immunoglobulin heavy-chain locus in developing B cells[J].Genes &Development,2005,19(3):322-327.
[35] BIAN X, WU L, MU L, et al.Spleen tyrosine kinase from Nile tilapia (Oreochromis niloticus): molecular characterization, expression pattern upon bacterial infection and the potential role in BCR signaling and inflammatory response[J].Fish &Shellfish Immunology, 2018, 82: 162-172.
[36] HAQSHENAS G,DOERIG C.Targeting of host cell receptor tyrosine kinases by intracellular pathogens[J].Science Signaling,2019,12(599):eaau9894.
[37] WEHRSTEDT S,KUBIS J,ZIMMERMANN A,et al.The tyrosine kinase inhibitor dasatinib reduces the growth of intracellular Mycobacterium tuberculosis despite impairing T-cell function[J].European Journal of Immunology,2018,48(11):1892-1903.
[38] CHENG Y X,XU W B,DONG W R,et al.Identification and functional analysis of epidermal growth factor receptor (EGFR) from Scylla paramamosain:the first evidence of two EGFR genes in animal and their involvement in immune defense against pathogen infection[J].Molecular Immunology,2022,151:143-157.