病原菌致病力与其携带的多种分泌系统密切相关,细菌可通过分泌系统将毒力蛋白或效应因子释放到外界环境中,或者直接作用于原核或真核生物,从而调控细菌与宿主的相互作用。在革兰氏阴性细菌中,分泌系统可分为两大类,分别为跨双层细胞膜分泌系统及单跨外膜分泌系统。其中有5种跨双层膜分泌系统类型已被鉴定,依次为T1SS、T2SS、T3SS、T4SS和T6SS(typeⅠ,Ⅱ,Ⅲ,Ⅳ,Ⅵ secretion systems)及1种单跨外膜分泌系统T5SS (type Ⅴ secretion system)[1]。Ⅵ型分泌系统(type Ⅵ secretion system,T6SS)于2006年从非O1和非O139血清型的霍乱弧菌(Vibrio cholerae)中被鉴定[2],至今依然是细菌分泌系统研究的热点。T6SS存在于约1/4的变形菌门细菌中,由15~20个核心组分构成[3]。T6SS具有多种生物学功能,包括介导细菌间的竞争、杀伤作用[4-5],以及调控毒力基因表达[6]、生物被膜形成与运动性[7]、胞外金属离子摄取[8]、鞭毛基因表达[9]和环境适应性[10]等。T6SS活性主要受群体感应系统[11]、磷酸化与去磷酸化修饰[12]及组蛋白样核结构蛋白[13]等胞内外信号因子调节。作为构成T6SS管状结构的核心蛋白,溶血素共调节蛋白(hemolysin coregulatory protein,Hcp)在效应因子分泌和T6SS装置组装的调控中发挥着关键作用。Hcp可为T6SS效应蛋白提供锚定位置,协助效应蛋白的折叠,同时也是一种可分泌到胞外的T6SS特征性效应蛋白[14]。Hcp还作为分子伴侣协助效应因子的折叠,并结合、输送相对分子质量为10 000~30 000的效应蛋白进行转运[15]。尽管T6SS及Hcp功能在不同物种之间具有相似性,然而,目前有关T6SS作用及其调控机制方面的研究,仍主要集中在临床环境中分离的各种人类病原菌,对水生动物致病菌T6SS及其核心组分的功能研究和认识还相对不足。本文围绕水生动物病原菌T6SS的生物学功能及其调节机制、溶血素共调节蛋白Hcp系统进化关系及其生物学功能的最新研究进展进行了阐述,以期为了解水生动物致病菌T6SS的研究概况和水生动物病害防控提供科学参考。
1 T6SS的结构与功能
冷冻透射电子显微镜分析显示,T6SS是一个噬菌体样注射器结构的复合物,以倒置的形式镶嵌于双层细胞膜上。T6SS通常由13个核心蛋白成分组成,包括构成分泌系统的跨膜复合结构和噬菌体样的穿刺结构(图1)。
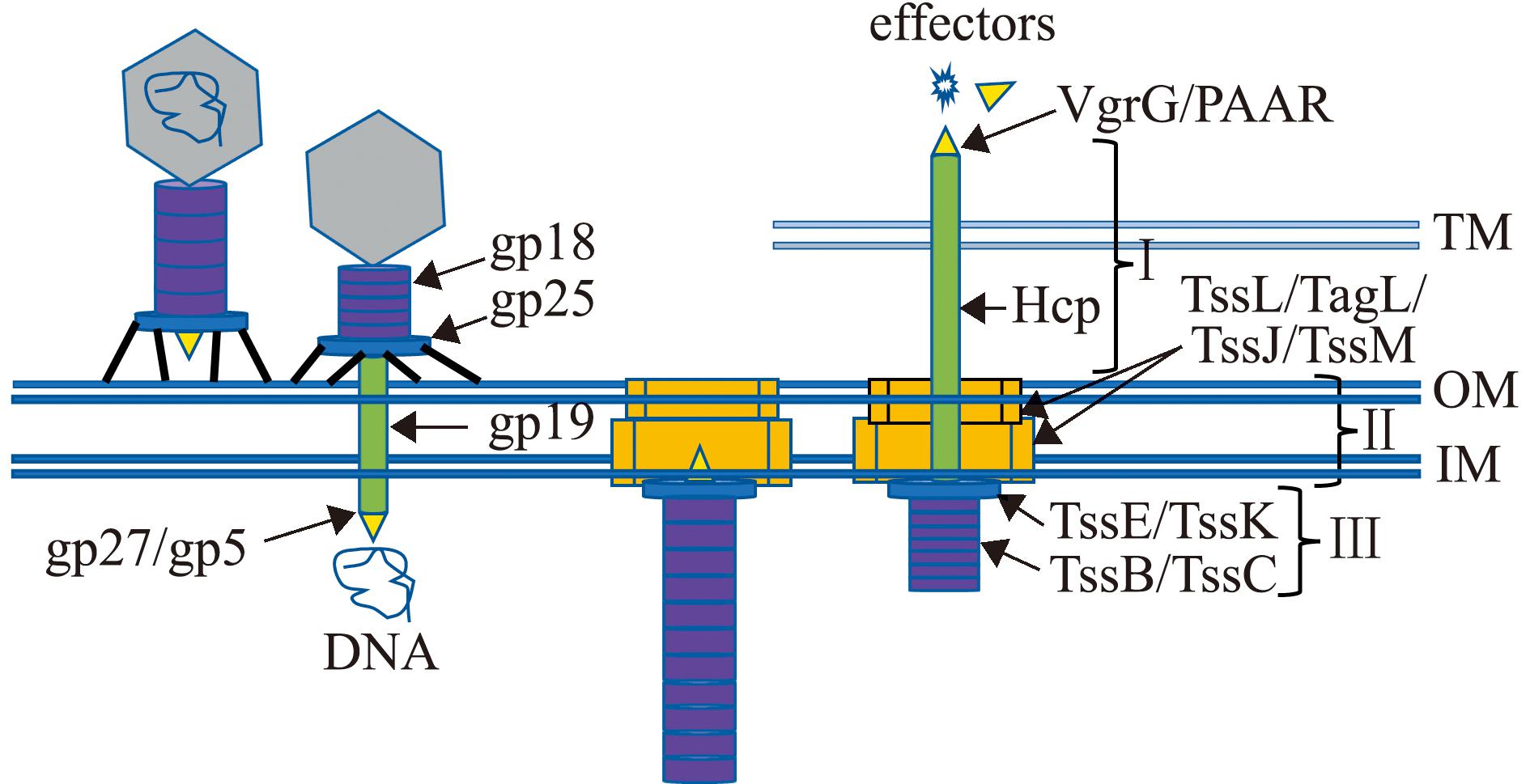
Ⅰ—穿刺结构;Ⅱ—膜复合体;Ⅲ—收缩鞘结构。依照参考文献[23]绘制。
Ⅰ—puncturing device;Ⅱ—membrane complex;Ⅲ—contractile sheath.According to the literature[23].
图1 T6SS与T4噬菌体结构示意图
Fig.1 Diagram of T6SS and T4 bacteriophage structures
1.1 T6SS的跨膜复合结构
T6SS跨膜复合结构由TssL、TagL、TssJ和TssM 4个膜蛋白形成。其中,TagL可穿过3个跨膜组分插入内膜,以类似于锚的结构将装置固定在细胞壁上;TssL是一种以钩状折叠的构象定位于细胞质的内膜蛋白;TagL与TssL可通过一个C端跨膜区域锚定在一起[16]。
1.2 噬菌体样的穿刺结构
T6SS基因簇可编码多种噬菌体样蛋白,如溶血素共调节蛋白Hcp(与噬菌体的gp19尾管蛋白同源),可形成内径约为4 nm的六元环,长达100 nm的管状结构是效应蛋白的转运通道[17];缬-甘氨酸重复蛋白G[valine-glycine repeat protein G(VgrG),与噬菌体的gp5和gp27顶端融合蛋白同源],位于Hcp管道末端,可形成三聚体的细胞穿刺结构,由Hcp管道推向靶细胞[18];TssB和TssC蛋白(类似于噬菌体的gp18),包围在Hcp尾管结构的外侧,形成一个类似于噬菌体收缩鞘结构[19],TssB/TssC可持续地进行组装、拆卸和回收等周期性循环[20];TssE蛋白(与噬菌体gp25基板组件蛋白同源),形成类似于噬菌体基板结构,与噬菌体gp25的功能类似,可能参与Hcp管道和TssB/TssC鞘结构的组装[21];脯氨酸(Proline)-丙氨酸(Alanine)-丙氨酸(Alanine)-精氨酸(Arginine)(PAAR)重复蛋白家族成员,位于VgrG顶端,形成一个顶端穿刺结构,PAAR与VgrG的相互作用可增加整个分泌装置的稳定性[22]。
1.3 与结构相关的功能作用
细菌T6SS跨膜复合结构与穿刺结构是决定其生物学功能的重要基础。跨膜复合结构是T6SS的平台基石,这些复合蛋白镶嵌在细菌双层细胞膜上,为T6SS功能发挥提供支撑作用。与功能较单一的跨膜复合结构不同,T6SS穿刺结构主要是为分泌系统提供能量和各种效应因子的分泌。研究发现,Hcp、VgrG和PAAR既是T6SS的结构性效应因子,也是其他效应分子经T6SS分泌至胞外的停泊位点[23]。其中,由Hcp形成的同源六聚体的管腔内侧存在不同的表位,可协同各种效应因子的输出,如肽聚糖水解酶Tse1、Tse3和RNA降解酶Tse2的分泌[24]。VgrG-PAAR复合物根据其功能可进一步分为VgrG-PAAR核心复合物和VgrG-PAAR辅助复合物[25]。T6SS效应分子输出的另一重要途径,便是与VgrG-PAAR针尖复合物的表位结合,如溶菌酶样效应蛋白TseP、TseC和DNA降解酶Rhs的分泌[26-27]。最近研究发现,接受效应因子停泊的结构蛋白可能并不是彼此独立发挥功能的。通过晶体结构分析表明,Hcp-VgrG-PAAR以α和β螺旋、氢键等形式形成一个内径为0.28 nm的传送系统,这样的结构更有利于细菌效应蛋白的快速输出[28]。
2 常见水生动物病原菌T6SS的生物学功能
2.1 副溶血性弧菌(Vibrio parahaemolyticus)
副溶血性弧菌是一种水生革兰氏阴性菌,在海洋和河口生态系统中普遍存在,可经海产品传播导致人类腹泻疾病,引起人类肠胃炎和伤口感染。首次大流行是由血清型O3:K6副溶血性弧菌引起的,此后,其他血清型引起的流行感染也越来越多地被报道[29-30]。基因组测序表明,副溶血性弧菌T6SS1和T6SS2分别位于1号和2号染色体[31],由毒力岛基因所编码[32]。副溶血弧菌T6SS1主要介导细菌在宿主细胞的黏附、定植和毒力产生[33],并通过分泌效应蛋白引发自噬反应从而诱导宿主发病,而T6SS2则介导细菌间的拮抗作用。[34-35]。副溶血弧菌主要产生两种与溶血和细胞毒性有关的毒力因子,即耐热性直接溶血素(TDH)和/或TDH相关溶血素(TRH)[36],副溶血弧菌的进化分析显示,T6SS可能涉及以上两种毒力因子的产生过程[37]。
2.2 嗜水气单胞菌(Aeromonas hydrophila)
嗜水气单胞菌在温水环境中普遍存在,主要引发鱼类气单胞菌败血症(motile aeromonas septicemia,MAS)[38]。研究发现,嗜水气单胞菌T6SS在不同区域来源的菌株中存在结构成分上的差异,如大多数来自美国的分离株不具有完整的T6SS,只编码了3个核心元件[tssD(Hcp)、tssH和tssI(VgrG)],而来自中国的分离株则具有完整的T6SS,但T6SS核心成分hcp1和vgrG1的敲除均可导致嗜水气单胞菌对斑点叉尾鮰(Ictalurus punctatus)幼鱼的毒力降低[39]。嗜水气单胞菌的毒力作用与VgrG在调节蛋白酶生成、菌活力及生物被膜形成方面有关[40]。效应蛋白-免疫因子的分泌是许多病原菌在发挥杀伤作用过程中为防止自身被误伤而采取的一种常见的T6SS分泌策略。研究表明,嗜水气单胞菌T6SS所分泌的一种磷脂酶Tle1AH,可促进生物被膜的形成,增强抗菌竞争能力及对斑马鱼的致病力;然而研究人员发现,在Tle1AH下游存在一种重要的同源免疫蛋白Tli1Tli2AH,可保护细菌自身免遭Tle1AH的攻击[41]。TseC-TsiC同样被证实是由T6SS所分泌并在嗜水气单胞菌的菌群竞争过程中发挥重要作用的效应蛋白-免疫因子对[42]。
2.3 溶藻弧菌(Vibrio alginolyticus)
溶藻弧菌广泛分布于海洋和河口环境中,是一种对海洋动物和人类均会构成潜在威胁的水生动物条件致病菌[43]。全基因组测序显示,溶藻弧菌可编码两套T6SS,即T6SS1和T6SS2。然而,目前有关T6SS1、T6SS2的功能作用未形成统一认识。鱼体竞争试验表明,溶藻弧菌EPGS株的两套T6SS均不参与宿主毒力作用,但T6SS2可介导细菌间的竞争和杀伤能力,而T6SS1则无此作用[44]。另有研究表明,溶藻弧菌VaT6SS1和VaT6SS2均可分泌MIX Ⅴ型(marker for type sIX effectors)抗细菌效应蛋白,这些效应蛋白被证实可在副溶血弧菌等海洋弧菌之间进行水平转移和交流,从而增强这些海洋弧菌与其他水生环境细菌的竞争作用[45]。
2.4 哈维氏弧菌(Vibrio harveyi)
哈维氏弧菌是一种革兰氏阴性嗜盐细菌,其基因组可编码3套T6SS(T6SS1、T6SS2、T6SS3)[46]。TssJ是哈维氏弧菌T6SS膜蛋白复合体成分之一,注射TssJ重组蛋白或DNA疫苗后的卵形鲳鲹(Trachinotus blochii)体内,碱性磷酸酶(AKP)、酸性磷酸酶(ACP)、溶菌酶(LZM)和超氧化物歧化酶(SOD)的活性显著增强,同时白介素10(IL10)、补体3(C3)、组织相容性复合体Iα与IIα(MHC Iα、MHC IIα)和免疫球蛋白M(IgM)的表达水平也明显上调,且在诱导鱼体产生血清抗体能力方面,TssJ重组蛋白免疫组要强于DNA疫苗免疫组[41]。此外,对T6SS主要效应因子rbsB、luxP、luxO、vgrG和vasC各敲除株的功能研究表明,哈维氏弧菌T6SS主要涉及细菌生物被膜形成、运动性及拮抗其他细菌的能力等[47]。
2.5 迟钝爱德华氏菌(Edwardsiella tarda)
迟钝爱德华氏菌(部分菌株也被称为杀鱼爱德华氏菌)是一种在世界范围内广泛流行,可引起多种经济养殖鱼类系统性出血性败血症和烂身等疾病的病原菌[48]。T6SS效应蛋白EvpP可介导迟钝爱德华氏菌侵袭宿主细胞[49]。EvpP通过与Hcp相互结合进行转运,是迟钝爱德华氏菌侵袭鱼类和产生毒力的重要致病因子[50-51]。细胞水平研究显示,EvpP致病机制可能与其抑制Ca2+依赖的Jnk途径及阻断NLRP3炎症小体的激活有关,这为细菌在胞内定植创造了有利条件[52-53]。而体内研究也证实,EvpP可降低斑马鱼Jnk信号通路的磷酸化水平,从而下调趋化因子Cxcl8a、基质金属肽酶Mmp13及炎症因子IL-1β的表达水平,并抑制中性粒细胞向感染部位的募集作用[54]。
2.6 变形假单胞菌(Pseudomonas plecoglossicida)
变形假单胞菌是一种温度依赖的条件性致病菌,低温时可引起大黄花鱼(Larimichthys crocea)内脏白色结节病[55]。变形假单胞菌基因组编码3套Ⅵ型分泌系统(T6SS1、T6SS2、T6SS3)。其中,T6SS1是主要毒力因子之一,T6SS2和T6SS3直接或间接参与致病性。T6SS1突变体在鱼脾脏中3 d内消失,而其他菌株持续增加,说明T6SS1主要调节体内细菌复制[56]。ClpV是变形假单胞菌T6SS分泌的一种ATP酶。斜带石斑鱼(Epinephelus coiodes)被clpV-RNAi菌株感染20 d内未观察到死亡;斜带石斑鱼脾脏、肾脏和肝脏在感染后5~8 d内均未出现明显结节,肿胀逐渐消失;与对照组相比,斜带石斑鱼的脾脏和血液在感染后的大部分时间点clpV-RNAi菌株的丰度均明显减少,头肾和躯干肾脏中clpV-RNAi菌株的丰度也从感染后72 h急剧下降[57]。由此可见,T6SS在变形假单胞菌的致病过程中发挥了重要的调控作用。
3 T6SS生物学功能的调节机制
3.1 群体感应
群体感应系统(quorum sensing system,QS)涉及细菌许多分泌系统和毒力相关因子的调控过程[58]。QS可调控T6SS的合成与分泌,从而影响其致病作用。副溶血弧菌OpaR和AphA是QS的核心调控因子,分别在菌体高密度和低密度时高表达。当细胞密度较低时,调控因子LuxO被激活,从而抑制OpaR活性并促进AphA的表达;在较高的细胞密度下,LuxO的活性受到限制,导致AphA活性的抑制和OpaR的激活[59];OpaR能抑制T6SS管状结构蛋白Hcp1的表达从而负调节T6SS1的活性,同时正调节T6SS2的活性[60]。作为副溶血弧菌膜结合的毒力调节蛋白ToxR与AphA、OpaR共同作用抑制T6SS1活性,而toxR基因的表达又与QS密切相关[61]。PpkA2激酶对底物的磷酸化介导了T6SS2和QS之间的相互作用,从而调控溶藻弧菌EPGS T6SS2的杀菌能力[44]。溶藻弧菌磷酸酶PppA由T6SS基因簇所编码,全基因转录组分析揭示了PppA存在多种调控靶点,包括T6SS底物溶血素共调节蛋白(Hcp)、群体感应调节因子LuxR、外毒素碱性丝氨酸蛋白酶(Asp)、鞭毛蛋白及多糖生物合成与转运蛋白,因此,PppA磷酸化作用是连接T6SS、c-di-GMP 产生及群体感应的桥梁[62]。群体感应还可通过调节特定的sigma (σ) 依赖性激活因子的表达从而调节T6SS的功能。如RpoE是一种选择性σ因子和环境适应调节因子,其受群体感应系统调节并影响变形假单胞菌T6SS的表达[63]。嗜水气单胞菌T6SS的功能也严格受到σ54转录激活因子VasH的调控[64]。
3.2 双组分调控和磷酸化/去磷酸化修饰
双组分调控和磷酸化/去磷酸化修饰是细菌响应胞内外信号的重要调节机制[65]。研究表明,迟钝爱德华氏菌T6SS所分泌的效应蛋白EvpP受到双组分系统EsrA-EsrB的正调控及组蛋白样核结构蛋白(H-NS)的负调控[66]。假单胞菌T6SS的组装和去组装过程分别受到丝氨酸/苏氨酸激酶(STK)和丝氨酸/苏氨酸磷酸酶(STP)通过Fha的磷酸化和去磷酸化作用所调控。正常生理条件下,PppA使铜绿假单胞菌Fha1磷酸化维持在较低水平,当PpkA感知到未知的环境信号时,该激酶会抑制PppA活性,Fha就会被磷酸化从而启动信号级联反应,进而激活T6SS的组装并发挥功能[67]。因此,PpkA/PppA/Fha1信号轴是翻译后磷酸化修饰水平调节T6SS活性的重要途径[68]。
3.3 温度及盐度
大部分海洋性病原菌T6SS活性均不同程度地受到环境温度的调节。在温暖的夏季,海洋细菌群体不断增加,副溶血弧菌利用活跃的T6SS1可以对抗其他不同种属海洋细菌和自身同种细菌,以利于在环境中生存[69]。溶藻弧菌T6SS活性受到盐度和温度的双重调控。其中,T6SS1在LB平板培养条件下,低盐度有利于其表达;而在MLB高盐培养条件下可激活T6SS2。溶藻弧菌T6SS对培养温度的敏感性也存在差异,其中在30 ℃培养条件下,有利于T6SS1和T6SS2的表达,而在37 ℃培养条件下,唯有T6SS2可保持活性[45]。变形假单胞菌各Hcp的分泌也受温度的调节,如Hcp1在12~28 ℃时分泌,Hcp2在12~35 ℃时分泌,而Hcp3在体外无分泌[56]。另外一项研究发现,变形假单胞菌的致病力在20℃培养条件下强于30 ℃,且在低温时Hcp的mRNA水平也高于高温培养时的mRNA水平。[70]。因此,低温条件有利于促进变形假单胞菌T6SS的表达从而增强其毒力[71]。
4 水生动物病原菌溶血素共调节蛋白Hcp进化关系及其生物学功能
4.1 Hcp进化关系及其功能位点
Hcp在T6SS功能发挥过程中起着重要作用,分析各水生动物致病菌Hcp的亲缘关系及其功能位点是了解其生物学作用的前提。本文通过Mega 5软件对水生动物病原菌基因hcp系统进化树进行分析,发现其具有以下两个特点:一是,除嗜水气单胞菌3个hcp亚型之间的进化关系较密切(爱德华氏菌除外,因其hcp亚型只有一个)外,其余各弧菌属病原菌hcp亚型在亲缘关系上较疏远;二是,爱德华氏菌与变形假单胞菌两者的hcp亲缘关系较接近(图2)。根据氨基酸序列,采用Softberry软件预测了不同水生动物病原菌Hcp蛋白的功能位点(图3),结果显示,病原菌Hcp蛋白功能位点有蛋白激酶C磷酸化位点、酪蛋白激酶Ⅱ磷酸化位点、酪氨酸激酶磷酸化位点、N-豆蔻酰化位点、异戊烯基结合位点、微生物羧基端定位信号位点、N-糖基化位点、cAMP-和cGMP-蛋白激酶磷酸化位点及ATP/GTP结合位点的基序A(P-环)等9种,其中,微生物羧基端定位信号位点是所有Hcp蛋白共同具有的功能位点。除了嗜水气单胞菌NJ-35和爱德华氏菌EIB202的3个Hcp亚型具有相同的功能位点外,其他病原菌各Hcp亚型之间功能位点均不同。从进化关系及其具有的蛋白功能位点方面进行比较,Hcp蛋白在溶藻弧菌与副溶血弧菌之间具有较高的同源性和相似性。尽管如此,这些位点对Hcp生物学功能的影响及其在T6SS致病过程中的作用机制鲜有报道。

最大似然法,自展值=1 000。
Bootstrap value is 1 000 by maximum composite likelihood method.
图2 水生动物病原菌溶血素共调节蛋白编码基因hcp的系统进化分析
Fig.2 Phylogenetic tree analysis of hcp genes encoding hemolysin co-regulatory protein of aquatic animal pathogenic bacteria

图3 水生动物病原菌溶血素共调节蛋白(Hcp)功能位点比较
Fig.3 Protein functional site comparison of hemolysin co-regulatory protein (Hcp) from aquatic animal pathogenic bacteria
4.2 Hcp生物学作用
作为最早被确认的T6SS核心组分,Hcp最初的功能被定义为构成T6SS管道分泌装置的必需结构蛋白[72]。随后研究发现,Hcp还充当效应蛋白或(和)分子伴侣的角色。目前研究显示,水生动物致病菌Hcp具有调节细菌的生物被膜形成、黏附、运动性、免疫逃逸和胞内存活等多种生物学功能。嗜水气单胞菌中国流行株NJ-35基因组可编码3个Hcp,其中,Hcp1是T6SS组装的必要条件,在细菌竞争中起主导作用;Hcp2对生物被膜的形成和细菌黏附具有负向调节作用,也涉及对斑马鱼毒力和面对四膜虫(Tetrahymena)捕食过程中存活能力的调控作用;而Hcp3则对生物被膜的形成和细菌黏附有正向调节作用[73]。这些结果表明,嗜水气单胞菌NJ-35 3个Hcp蛋白参与了该菌环境适应性和毒力调控的不同过程。位于嗜水气单胞菌SSU株 T6SS基因簇内的Hcp2是T6SS装置的结构蛋白,而位于染色体远端位置的Hcp1则更多发挥着效应蛋白的作用;小鼠感染模型显示,只有Hcp1负调控细菌的运动性和蛋白酶的产生,而Hcp1、Hcp2蛋白均是嗜水气单胞菌扩散到外周器官所依赖的毒力因子[40]。这种Hcp功能的非冗余性同样体现在爱德华氏菌。爱德华氏菌是一种可引起斑点叉尾鮰肠败血症(Enteric septicemia of catfish,ESC)的革兰氏阴性兼性细胞内病原体;Hcp1或Hcp1/Hcp2缺失后,爱德华氏菌对斑点叉尾鮰的毒力作用明显减弱;进一步的体内外试验表明,Hcp1主要参与该菌在斑点叉尾鮰体内的致病作用,而Hcp2与该菌在斑点叉尾鮰巨噬细胞与上皮细胞的黏附和存活作用有关[74]。此外,针对Hcp所构建的重组疫苗也显示出了对特定病原菌感染的预防作用。如接种嗜水气单胞菌Hcp重组蛋白的鲤(Cyprinus carpio)被细菌感染后,存活率显著提高(46.67%),免疫鱼血清中IgM抗体水平显著升高,肾、脾和鳃中白细胞介素-1β (IL-1β)和肿瘤坏死因子-α (TNF-α) 等细胞因子水平也显著升高[75],因此,Hcp重组蛋白有望成为抗病原菌感染的候选疫苗。
5 存在问题及展望
近年来,通过采用冷冻电镜技术、基因敲除及免疫沉淀等分子生物学方法,人们对T6SS结构和功能的研究逐渐深入。然而,当前大部分研究结论均来自临床分离菌株,对水生动物致病菌T6SS及其核心组分的研究相对薄弱,主要体现在对T6SS及其核心组分引起水产养殖动物致病机制研究的匮乏,以及不同致病菌间T6SS研究的不充分,具体存在问题及未来研究方向建议如下。
5.1 水生动物致病菌T6SS研究存在的问题
1)致病机制研究不够深入。目前,T6SS及其核心组分在霍乱弧菌、大肠杆菌及绿脓假单胞菌等引起人类致病的机制研究中较为多见,但这些组分在水生动物致病菌中的作用是否存在并不清楚。有关水产养殖领域较常见病原菌T6SS的功能机制研究还处于起步阶段。
2)不同致病菌T6SS的研究不够充分。目前,水生动物致病菌T6SS研究主要集中在嗜水气单胞菌,而对副溶血弧菌、哈维氏弧菌和溶藻弧菌等水生动物致病菌的研究报道较少。此外,一些已被报道的T6SS功能还存在一些争议,如溶藻弧菌T6SS的杀菌和杀真核生物功能等,故需要更多的试验数据支持。
5.2 未来研究方向
针对以上问题和不足,今后工作可从以下几方面展开。
1)深入挖掘和全面了解水生动物病原菌T6SS的生物学功能。利用现代分子生物学技术构建体内外感染模型,重点研究其对水产养殖动物的致病机制,包括黏附力、侵袭力、胞内存活、毒力因子分泌和免疫逃逸等,以期寻找出不同水生动物病原菌T6SS保守的分子机制。
2)加强对T6SS及其组分活性调控与环境因素的关联性研究。水生动物病原菌T6SS及其组分的表达受到温度和盐度的调节,探索致病菌T6SS受温度和盐度调节的信号分子及其响应机制,对新型防控策略的制定具有重要现实意义。
3)拓展T6SS及其组分与致病菌代谢之间的调控关系研究。细菌的致病力与其代谢息息相关,T6SS除具有分泌效应蛋白功能外,还涉及胞外营养摄取,由此推测,T6SS可能参与细菌的代谢过程。而本课题组前期转录组学和蛋白质组学研究显示,溶藻弧菌T6SS与其碳氮代谢和内毒素产生存在关联。然而,具体调控机制有待进一步探究。
综上所述,探索水生动物病原菌T6SS及其核心组分Hcp在不同种属之间的保守性和功能机制,重点围绕水生动物病原菌毒力调节的环境驱动机制、细菌代谢与致病力之间的调控等方面开展研究,可为药物开发和疫苗研制提供新的靶点,同时也可为更好地开展鱼类细菌性疫病防控工作提供理论支撑。
[1] COSTA T R D,FELISBERTO-RODRIGUES C,MEIR A,et al.Secretion systems in Gram-negative bacteria:structural and mechanistic insights[J].Nature Reviews Microbiology,2015,13(6):343-359.
[2] PUKATZKI S,MA A T,STURTEVANT D,et al.Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system[J].Proceedings of the National Academy of Sciences of the United States of America,2006,103(5):1528-1533.
[3] ZHANG J,GUAN J,WANG M,et al.SecReT6 update: a comprehensive resource of bacterial Type Ⅵ Secretion Systems[J]. Science China Life Sciences,2023,66(3):626-634.
[4] JANA B,SALOMON D.Type Ⅵ secretion system:a modular toolkit for bacterial dominance[J].Future Microbiology,2019,14:1451-1463.
[5] ROBITAILLE S,TRUS E,ROSS B D.Bacterial defense against the type Ⅵ secretion system[J].Trends in Microbiology,2021,29(3):187-190.
[6] FERIA J M,VALVANO M A.An overview of anti-eukaryotic T6SS effectors[J].Frontiers in Cellular and Infection Microbiology,2020,10:584751.
[7] LI Y Q,CHEN L,ZHANG P S,et al.ClpV3 of the H3-type Ⅵ secretion system (H3-T6SS) affects multiple virulence factors in Pseudomonas aeruginosa[J].Frontiers in Microbiology,2020,11:1096.
[8] 刘琬洋,权国梅,刘家奇,等.革兰氏阴性菌Ⅵ型分泌系统及其参与金属离子转运的研究进展[J].微生物学报,2021,61(11):3377-3390.
LIU W Y,QUAN G M,LIU J Q,et al.Advances of the Gram-negative bacterial type Ⅵ secretion system and its function for metal ion acquisition[J].Acta Microbiologica Sinica,2021,61(11):3377-3390.(in Chinese)
[9] BOUTEILLER M,GALLIQUE M,BOURIGAULT Y,et al.Crosstalk between the type Ⅵ secretion system and the expression of class IV flagellar genes in the Pseudomonas fluorescens MFE01 strain[J].Microorganisms,2020,8(5):622.
[10] YU K W,XUE P,FU Y,et al.T6SS mediated stress responses for bacterial environmental survival and host adaptation[J].International Journal of Molecular Sciences,2021,22(2):478.
[11] PENA R T,BLASCO L,AMBROA A,et al.Relationship between quorum sensing and secretion systems[J].Frontiers in Microbiology,2019,10:1100.
[12] ZIVERI J,CHHUON C,JAMET A,et al.Critical role of a sheath phosphorylation site on the assembly and function of an atypical type Ⅵ secretion system[J].Molecular &Cellular Proteomics:MCP,2019,18(12):2418-2432.
[13] CUI S L,XIAO J F,WANG Q Y,et al.H-NS binding to evpB and evpC and repressing T6SS expression in fish pathogen Edwardsiella piscicida[J].Archives of Microbiology,2016,198(7):653-661.
[14] GAL N J E,WAKSMAN G.Protein-injection machines in bacteria[J].Cell,2018,172(6):1306-1318.
N J E,WAKSMAN G.Protein-injection machines in bacteria[J].Cell,2018,172(6):1306-1318.
[15] HOWARD S A,FURNISS R C D,BONINI D,et al.The breadth and molecular basis of Hcp-driven type Ⅵ secretion system effector delivery[J].mBio,2021,12(3):e0026221.
[16] STIETZ M S,LIANG X Y,LI H,et al.TssA-TssM-TagA interaction modulates type Ⅵ secretion system sheath-tube assembly in Vibrio cholerae[J].Nature Communications,2020,11:5065.
[17] NOREEN Z,JOBICHEN C,ABBASI R,et al.Structural basis for the pathogenesis of Campylobacter jejuni Hcp1,a structural and effector protein of the type Ⅵ secretion system[J].The FEBS Journal,2018,285(21):4060-4070.
[18] LEIMAN P G,BASLER M,RAMAGOPAL U A,et al.Type Ⅵ secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin[J].Proceedings of the National Academy of Sciences of the United States of America,2009,106(11):4154-4159.
[19] BÖNEMANN G,PIETROSIUK A,DIEMAND A,et al.Remodelling of VipA/VipB tubules by ClpV-mediated threading is crucial for type Ⅵ protein secretion[J].The EMBO Journal,2009,28(4):315-325.
[20] BASLER M,PILHOFER M,HENDERSON G P,et al.Type Ⅵ secretion requires a dynamic contractile phage tail-like structure[J].Nature,2012,483(7388):182-186.
[21] SILVERMAN J M,BRUNET Y R,CASCALES E,et al.Structure and regulation of the type Ⅵ secretion system[J].Annual Review of Microbiology,2012,66:453-472.
[22] SHNEIDER M M,BUTH S A,HO B T,et al.PAAR-repeat proteins sharpen and diversify the type Ⅵ secretion system spike[J].Nature,2013,500(7462):350-353.
[23] HO B T,DONG T G,MEKALANOS J J.A view to a kill:the bacterial type Ⅵ secretion system[J].Cell Host &Microbe,2014,15(1):9-21.
[24] SILVERMAN J M,AGNELLO D M,ZHENG H J,et al.Haemolysin coregulated protein is an exported receptor and chaperone of type Ⅵ secretion substrates[J].Molecular Cell,2013,51(5):584-593.
[25] LIANG X Y,ZHENG H Y,ZHAO Y J,et al.VgrG spike dictates PAAR requirement for the assembly of the type Ⅵ secretion system[J].Journal of Bacteriology,2023,205(2):e0035622.
[26] LIANG X Y,PEI T T,LI H,et al.VgrG-dependent effectors and chaperones modulate the assembly of the type Ⅵ secretion system[J].PLoS Pathogens,2021,17(12):e1010116.
[27] GÜNTHER P,QUENTIN D,AHMAD S,et al.Structure of a bacterial Rhs effector exported by the type Ⅵ secretion system[J].PLoS Pathogens,2022,18(1):e1010182.
[28] HE W B,WU K,OUYANG Z L,et al.Structure and assembly of type Ⅵ secretion system cargo delivery vehicle[J].Cell Reports,2023,42(7):112781.
[29] LE N-SICAIROS N,ZATARAIN-LOPEZ R,ANGULO-ZAMUDIO U A,et al.Vibrio parahaemolyticus is associated with diarrhea cases in Mexico,with a dominance of pandemic O3:K6 clones[J].International Journal of Environmental Research and Public Health,2022,19(16):10318.
N-SICAIROS N,ZATARAIN-LOPEZ R,ANGULO-ZAMUDIO U A,et al.Vibrio parahaemolyticus is associated with diarrhea cases in Mexico,with a dominance of pandemic O3:K6 clones[J].International Journal of Environmental Research and Public Health,2022,19(16):10318.
[30] LI L Z,MENG H M,GU D,et al.Molecular mechanisms of Vibrio parahaemolyticus pathogenesis[J].Microbiological Research,2019,222:43-51.
[31] LI P,KINCH L N,RAY A,et al.Acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus strains maintain an antibacterial type Ⅵ secretion system with versatile effector repertoires[J].Applied and Environmental Microbiology,2017,83(13):e00737-e00717.
[32] IZUTSU K,KUROKAWA K,TASHIRO K,et al.Comparative genomic analysis using microarray demonstrates a strong correlation between the presence of the 80-kilobase pathogenicity island and pathogenicity in Kanagawa phenomenon-positive Vibrio parahaemolyticus strains[J].Infection and Immunity,2008,76(3):1016-1023.
[33] PAZHANI G P,CHOWDHURY G,RAMAMURTHY T.Adaptations of Vibrio parahaemolyticus to stress during environmental survival,host colonization,and infection[J].Frontiers in Microbiology,2021,12:737299.
[34] YU Y,FANG L H,ZHANG Y,et al.VgrG2 of type Ⅵ secretion system 2 of Vibrio parahaemolyticus induces autophagy in macrophages[J].Frontiers in Microbiology,2015,6:168.
[35] TCHELET D,KEPPEL K,BOSIS E,et al.Vibrio parahaemolyticus T6SS2 effector repertoires[J].Gut Microbes,2023,15(1):2178795.
[36] ZHONG X J,PAN Z H,MU Y J,et al.Characterization and epidemiological analysis of Vibrio parahaemolyticus isolated from different marine products in East China[J].International Journal of Food Microbiology,2022,380:109867.
[37] BOYD E F,COHEN A L V,NAUGHTON L M,et al.Molecular analysis of the emergence of pandemic Vibrio parahaemolyticus[J].BMC Microbiology,2008,8:110.
[38] RAHMAN M M,RAHMAN M A,HOSSAIN M T,et al.Efficacy of bi-valent whole cell inactivated bacterial vaccine against motile Aeromonas septicemia (MAS) in cultured catfishes (Heteropneustes fossilis,Clarias batrachus and Pangasius pangasius) in Bangladesh[J].Saudi Journal of Biological Sciences,2022,29(5):3881-3889.
[39] TEKEDAR H C,ABDELHAMED H,KUMRU S,et al.Comparative genomics of Aeromonas hydrophila secretion systems and mutational analysis of Hcp1 and VgrG1 genes from T6SS[J].Frontiers in Microbiology,2019,9:3216.
[40] SHA J,ROSENZWEIG J A,KOZLOVA E V,et al.Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis[J].Microbiology,2013,159(Pt 6):1120-1135.
[41] MA S Y,DONG Y H,WANG N N,et al.Identification of a new effector-immunity pair of Aeromonas hydrophila type Ⅵ secretion system[J].Veterinary Research,2020,51(1):71.
[42] LIANG X Y,MOORE R,WILTON M,et al.Identification of divergent type Ⅵ secretion effectors using a conserved chaperone domain[J].Proceedings of the National Academy of Sciences of the United States of America,2015,112(29):9106-9111.
[43] XIE J S,BU L F,JIN S,et al.Outbreak of vibriosis caused by Vibrio harveyi and Vibrio alginolyticus in farmed seahorse hippocampus kuda in China[J].Aquaculture,2020,523:735168.
[44] YANG Z,ZHOU X H,MA Y,et al.Serine/threonine kinase PpkA coordinates the interplay between T6SS2 activation and quorum sensing in the marine pathogen Vibrio alginolyticus[J].Environmental Microbiology,2018,20(2):903-919.
[45] SALOMON D,KLIMKO J A,TRUDGIAN D C,et al.Type Ⅵ secretion system toxins horizontally shared between marine bacteria[J].PLoS Pathogens,2015,11(8):e1005128.
[46] SUN Y,DING S S,HE M W,et al.Construction and analysis of the immune effect of Vibrio harveyi subunit vaccine and DNA vaccine encoding TssJ antigen[J].Fish &Shellfish Immunology,2020,98:45-51.
[47] 贝蕾.哈维弧菌T6SS中rbsB、vgrG、hcp基因的表达及功能分析[D].上海:上海海洋大学,2018.
BEI L.RbsB,vgrG,hcp gene of type Ⅵ secretion system(T6SS) in Vibrio harveyi:expression and functional analysis[D].Shanghai:Shanghai Ocean University,2018.(in Chinese)
[48] PARK S B,AOKI T,JUNG T S.Pathogenesis of and strategies for preventing Edwardsiella tarda infection in fish[J].Veterinary Research,2012,43(1):67.
[49] ZHANG J,XIAO J,ZHANG Y,et al.A new target for the old regulator:H-NS suppress T6SS secretory protein EvpP,the major virulence factor in the fish pathogen Edwardsiella tarda[J].Letters in Applied Microbiology,2014,59(5):557-564.
[50] WANG X,WANG Q Y,XIAO J F,et al.Edwardsiella tarda T6SS component evpP is regulated by esrB and iron,and plays essential roles in the invasion of fish[J].Fish &Shellfish Immunology,2009,27(3):469-477.
[51] HU W T,ANAND G,SIVARAMAN J,et al.A disordered region in the EvpP protein from the type Ⅵ secretion system of Edwardsiella tarda is essential for EvpC binding[J].PLoS One,2014,9(11):e110810.
[52] ZHANG L Z,NI C S,XU W T,et al.Intramacrophage infection reinforces the virulence of Edwardsiella tarda[J].Journal of Bacteriology,2016,198(10):1534-1542.
[53] CHEN H,YANG D H,HAN F J,et al.The bacterial T6SS effector EvpP prevents NLRP3 inflammasome activation by inhibiting the Ca2+-dependent MAPK-jnk pathway[J].Cell Host &Microbe,2017,21(1):47-58.
[54] TAN J C,YANG D H,WANG Z,et al.EvpP inhibits neutrophils recruitment via Jnk-caspy inflammasome signaling in vivo[J].Fish &Shellfish Immunology,2019,92:851-860.
[55] LI C W,WANG S L,REN Q L,et al.An outbreak of visceral white nodules disease caused by Pseudomonas plecoglossicida at a water temperature of 12 ℃ in cultured large yellow croaker (Larimichthys crocea) in China[J].Journal of Fish Diseases,2020,43(11):1353-1361.
[56] JIN J M,LI Y Y,HUANG M X,et al.Preliminary studies on the different roles of T6SSs in pathogenicity of Pseudomonas plecoglossicida NB2011[J].Journal of Fish Diseases,2021,44(11):1669-1679.
[57] LUO G,XU X J,ZHAO L M,et al.ClpV is a key virulence gene during in vivo Pseudomonas plecoglossicida infection[J].Journal of Fish Diseases,2019,42(7):991-1000.
[58] PENA R T,BLASCO L,AMBROA A,et al.Relationship between quorum sensing and secretion systems[J].Frontiers in Microbiology,2019,10:1100.
[59] KALBURGE S S,CARPENTER M R,ROZOVSKY S,et al.Quorum sensing regulators are required for metabolic fitness in Vibrio parahaemolyticus[J].Infection and Immunity,2017,85(3):e00930-e00916.
[60] MA L Z,ZHANG Y Q,YAN X J,et al.Expression of the type Ⅵ secretion system 1 component Hcp1 is indirectly repressed by OpaR in Vibrio parahaemolyticus[J].The Scientific World Journal,2012,2012:982140.
[61] ZHANG Y Q,GAO H,OSEI-ADJEI G,et al.Transcriptional regulation of the type Ⅵ secretion system 1 genes by quorum sensing and ToxR in Vibrio parahaemolyticus[J].Frontiers in Microbiology,2017,8:2005.
[62] SHENG L L,LV Y Z,LIU Q,et al.Connecting type Ⅵ secretion,quorum sensing,and c-di-GMP production in fish pathogen Vibrio alginolyticus through phosphatase PppA[J].Veterinary Microbiology,2013,162(2/3/4):652-662.
[63] ZHANG Y B,HUANG Y P,DING H Y,et al.A σE-mediated temperature gauge orchestrates type Ⅵ secretion system,biofilm formation and cell invasion in pathogen Pseudomonas plecoglossicida[J].Microbiological Research,2023,266:127220.
[64] LI J H,WU Z H,WU C S,et al.VasH contributes to virulence of Aeromonas hydrophila and is necessary to the T6SS-mediated bactericidal effect[J].Frontiers in Veterinary Science,2021,8:793458.
[65] GROISMAN E A.Feedback control of two-component regulatory systems[J].Annual Review of Microbiology,2016,70:103-124.
[66] WANG X,WANG Q Y,XIAO J F,et al.Hemolysin EthA in Edwardsiella tarda is essential for fish invasion in vivo and in vitro and regulated by two-component system EsrA-EsrB and nucleoid protein HhaEt[J].Fish &Shellfish Immunology,2010,29(6):1082-1091.
[67] WU Y J,GONG J Y,LIU S,et al.Crystal structure of PppA from Pseudomonas aeruginosa,a key regulatory component of type Ⅵ secretion systems[J].Biochemical and Biophysical Research Communications,2019,516(1):196-201.
[68] CASABONA M G,SILVERMAN J M,SALL K M,et al.An ABC transporter and an outer membrane lipoprotein participate in posttranslational activation of type Ⅵ secretion in Pseudomonas aeruginosa[J].Environmental Microbiology,2013,15(2):471-486.
[69] SALOMON D,GONZALEZ H,UPDEGRAFF B L,et al.Vibrio parahaemolyticus type Ⅵ secretion system 1 is activated in marine conditions to target bacteria,and is differentially regulated from system 2[J].PLoS One,2013,8(4):e61086.
[70] TAO Z,ZHOU T,ZHOU S M,et al.Temperature-regulated expression of type Ⅵ secretion systems in fish pathogen Pseudomonas plecoglossicida revealed by comparative secretome analysis[J].FEMS Microbiology Letters,2016,363(22):fnw261.
[71] HUANG L X,LIU W J,JIANG Q L,et al.Integration of transcriptomic and proteomic approaches reveals the temperature-dependent virulence of Pseudomonas plecoglossicida[J].Frontiers in Cellular and Infection Microbiology,2018,8:207.
[72] MOUGOUS J D,CUFF M E,RAUNSER S,et al.A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus[J].Science,2006,312(5779):1526-1530.
[73] WANG N N,LIU J,PANG M D,et al.Diverse roles of Hcp family proteins in the environmental fitness and pathogenicity of Aeromonas hydrophila Chinese epidemic strain NJ-35[J].Applied Microbiology and Biotechnology,2018,102(16):7083-7095.
[74] KALINDAMAR S,ABDELHAMED H,KORDON A,et al.Hemolysin co-regulated family proteins Hcp1 and Hcp2 contribute to Edwardsiella ictaluri pathogenesis[J].Frontiers in Veterinary Science,2021,8:681609.
[75] WANG N N,WU Y F,PANG M D,et al.Protective efficacy of recombinant hemolysin co-regulated protein (Hcp) of Aeromonas hydrophila in common carp (Cyprinus carpio)[J].Fish &Shellfish Immunology,2015,46(2):297-304.