有机磷酸酯(organophosphate esters,OPEs)是一类具有热稳定性、高沸点的新型人工合成化合物,其作为阻燃剂被广泛添加于电子器件、儿童玩具和家具建材等工业产品中。随着传统溴代阻燃剂被逐渐淘汰和禁止使用,OPEs作为溴代阻燃剂的理想替代物,生产和使用量快速增长[1]。据统计,1992年全球OPEs的使用量约为10万t,2018年增长到105万t[2]。中国是OPEs生产和使用大国,2007年OPEs使用量约为7万t,2013年迅速增加到30万t,随着国内生产制造业的迅速发展,预计OPEs的使用量会以每年15%的速度增长[2-3]。
由于OPEs是通过物理混合而非化学键合的形式与产品材料结合,因此,其容易随产品老化和磨损释放于环境中,并通过空气蒸发、雨水沉降和地表径流等途径进入水体中。在地中海西北部的马赛湾[4]、日本东京湾[5]等国外海水水体中OPEs均被大量检出。国内研究显示,OPEs在太湖[6]、黄海[7]、长江[8]和南海[9]等主要水产养殖水域中普遍被检出且污染水平呈逐年增长的趋势[10],OPEs已成为中国水产养殖环境中一种普遍存在的污染物。水环境中的OPEs通过饵料摄入、皮肤接触和呼吸等途径富集于水生生物中,不仅对水生生物具有神经发育毒性[11-13]、生殖发育毒性[14-15]及致癌性[16-17]等危害,还会引发一系列水产品质量安全问题。目前,水产品的OPEs污染受到国内外学者普遍关注,如Hou等[18]对北京周边河流中野生鲢、鲫和泥鳅中OPEs残留进行测定,发现样品中∑8OPEs的含量为265~1 973 ng/g(脂质量);Liu等[19]对珠江三角洲捕获的274个鱼类样本测定显示,鱼体中12种OPEs的含量为2.3~30.0 ng/g(湿质量);Bekele等[20]在辽州湾野生海鱼中检测到∑17OPEs的含量高达107 ng/g(干质量);Giulivo等[21]在欧洲埃夫罗塔斯河、阿迪杰河和萨瓦河流域捕捞的水生生物样品中检出OPEs含量为14.4~650.0 ng/g(脂质量),说明水产品中OPEs污染已经成为了全球性问题。然而,现阶段OPEs污染研究主要聚焦在环境领域,针对水产品的研究较为匮乏且研究对象普遍以野生捕捞水产品为主,关于养殖水产品中OPEs污染的研究鲜有报道。作为居民消费水产品的主要来源,2020年广东省养殖水产品产量占水产品总量的85.2%。水产品质量安全与居民健康密切相关[22],因此,分析养殖水产品中OPEs的污染水平,能更准确地反映其在水产品中残留引起的居民膳食健康风险情况。
红海湾海域是广东省传统海水养殖区,毗邻珠江口经济发达地区和电子产品生产及拆解业发达的粤东地区。近年来,邻近水域中的OPEs污染研究屡有报道[23-24],这提示红海湾海域水产品存在OPEs污染的潜在风险。本研究中,以红海湾海域养殖和捕捞水产品为研究对象,分析了使用量较大的10种商用OPEs(记为∑10OPEs)在不同栖息习性水生生物中的污染水平、组成特征和分布特征,评估了水产品中OPEs残留引起的人体健康风险,以期为水产品中OPEs污染物的管控和质量安全评估提供数据支持和参考依据。
1 材料与方法
1.1 材料
试验用水产品分别采自广东汕尾红海湾海域养殖和捕捞区。
仪器设备:气相色谱三重四级杆串联质谱仪(美国Agilent,GC-MS/MS,7890A-7000),配电子轰击离子源(EI源)和Agilent DB-5MS色谱柱(30 m×250 μm,膜厚0.25 μm);氮吹仪(美国Orgnomation,N-EVAP116);低温冷冻离心机(上海安亭,Anke DL-6000B);真空冷冻干燥机(德国Christ,ALPHA2-4LSC);旋涡混合器(德国IKA Works GmbH&Co,IKA MS3 Basic);马弗炉(日本Yamato,FO510C);粉碎机(天津泰斯特,FW100)。
1.2 方法
1.2.1 样品采集 2022年9月在汕尾红海湾海域近岸不同养殖场网箱内采集7个养殖品种共12个样品,每个样品由同一养殖网箱的3尾同一品种鱼类混合制备而成,主要养殖品种金鲳(Trachinotus blochii)在不同养殖网箱采集3个样品,花尾胡椒鲷(Plectorhinchus cinctus)、星点笛鲷(Lutjanus stellatus)和青石斑鱼(Epinephelus awoara)各采集2个样品,其余3个品种均采集1个样品。
在码头采集捕捞的马鲛(Scomberomorus niphonius)、金线鱼(Nemipterus virgatus)、龙头鱼(Harpadon nehereus)等16个品种共18个样品,其中,鲐(Scomber japonicus)和龙头鱼在不同码头采集到2个样品,其余品种均只采集到1个样品。样品采集当天冷藏运回实验室,取背侧肌和腹侧肌约200 g,冷冻干燥48 h后计算每份样品的含水率。冻干样品用粉碎机粉碎均质后过孔径为425 μm的筛,然后装入封口铝箔袋中,于-18 ℃下保存待分析。
1.2.2 样品前处理 称取0.50 g样品到50 mL玻璃离心管中,加入50 μL内标混合液(1 μg/mL)。向离心管中添加3 g无水硫酸钠和10 mL正己烷,涡旋混匀2 min,超声处理30 min后在4 ℃条件下以4 000 r/min离心10 min,将上清液转移到新的50 mL玻璃离心管中。样品重复提取2次,合并提取液。
将样品提取液过NH2固相萃取柱后用3 mL正己烷淋洗固相萃取柱,再用6 mL二氯甲烷与乙酸乙酯的混合溶液(二者体积比为1∶1)洗脱目标物,收集洗脱液用氮气浓缩至近干后,用1 mL乙酸乙酯定容待测。
1.2.3 试剂的配制 磷酸三(2-丁氧基乙基)酯[tris (2-butoxyethyl) phosphate,TBOEP,CAS:78-51-3]、磷酸三(2-氯乙基)酯[tris (2-chloroethyl) phosphate,TCEP,CAS:115-96-8]、磷酸三(2-氯异丙基)酯[tris (2-chloroisopropyl) phosphate,TCIPP,CAS:13674-84-5]、磷酸三苯酯(triphenyl phosphate,TPHP,CAS:115-86-6)、磷酸三正丁酯(tri-n-butyl phosphate,TNBP,CAS:126-73-8)、2-乙基己基二苯基磷酸酯(2-ethylhexyl diphenyl phosphate,EHDPP,CAS:1241-94-7)、磷酸三(1,3-二氯异丙基)酯[tris (1,3-dichloro-2-propyl) phosphate,TDCIPP,CAS:13674-87-8]、磷酸三(2-乙基己基)酯[tris (2-ethylhexyl) phosphate,TEHP,CAS:78-42-2]、磷酸三丙酯(tri-n-propyl phosphate,TNPP,CAS:513-08-6)和磷酸三乙酯(triethyl phosphate,TEP,CAS:78-40-0)10种OPEs标准品购于德国Dr.Ehrenstorfer公司,纯度为92.0%~99.6%。10种OPEs标准品用乙酸乙酯配制成浓度为100 μg/mL的标准贮备液并于-18 ℃下避光保存。磷酸三乙酯-d15(triethyl phosphate-d15,TEP-d15,CAS:135942-11-9)和磷酸三苯酯-d15(triphenyl phosphate-d15,TPHP-d15,CAS:1173020-30-8)购自挪威Chiron公司,纯度≥99%;磷酸三正丁酯-d27(tri-n-butyl phosphate-d27,TNBP-d27,CAS:61196-26-7)购自加拿大Toronto Research Chemicals公司,纯度为98%。3种氘代内标物质用乙酸乙酯配制成浓度为100 μg/mL的标准贮备液并于-18 ℃下避光保存。
色谱级有机溶剂二氯甲烷、甲醇和乙酸乙酯购自默克公司(Merck),正己烷购自J.T.Baker公司;无水硫酸钠(优级纯,纯度99%)购自麦克林公司(Macklin),使用前在马弗炉中450 ℃下烘烤4 h;NH2固相萃取柱(500 mg/3cc)购自Waters公司,使用前用3 mL二氯甲烷与乙酸乙酯混合液(二者体积比为1∶1)和3 mL正己烷预活化。
1.2.4 仪器测定条件 进样口温度为280 ℃,采用不分流模式进样,进样量为1 μL;载气(氦气,纯度≥99.999%)流速为1.2 mL/min。升温程序为:初始柱温70 ℃保持1 min,以20 ℃/min升温至280 ℃后,再以30 ℃/min升温至300 ℃,保持3 min。离子源(EI源)电子能量为70 eV;离子源、四级杆和传输线温度分别为250、150、280 ℃。碰撞气(氮气,纯度≥99.999%)和淬灭气(氦气,纯度≥99.999%)流速分别为1.5、2.25 mL/min。采用多反应监测模式,监测离子使用相关报道中的OPEs监测离子[25]。
1.2.5 质量控制 玻璃离心管在使用前置于马弗炉中450 ℃下处理 4 h。以基质加标校准曲线对生物样品中的OPEs进行定量分析,曲线浓度范围为1~200 ng/g时,各化合物线性相关系数范围为0.998 3~0.999 9,10种OPEs的方法检测限范围为0.03~0.47 ng/g,回收率范围为80.6%~128.6%;每批次样品均设置试剂空白,检测结果扣除试剂空白;进样时每隔10个样品进一针标准溶液,以监控批量进样时仪器的稳定性。
1.2.6 风险评估模型 OPEs非致癌风险评估采用危害指数(hazard index,HI)模型[26-27],计算公式为
IHI=∑(IEDI×10-6)/IRfD,
(1)
IEDI=IDC×Ci/WBW。
(2)
式中:IHI为危害指数;IRfD(reference dose)为各OPE化合物经口摄入的参考剂量[mg/(kg·d)],其值使用Xing等[8]和Luo等[28]的数据(表1);IEDI(estimated daily intake)为居民每日各OPEs的估计摄入量[ng/(kg·d)];IDC (daily consumption)为居民每日水产品的摄入量(g/d);Ci(concentration)为各OPE化合物在水产品中的平均浓度(ng/g 湿质量);WBW(body weight)为居民平均体质量(kg)。不同年龄段男性和女性的平均体质量及每日水产品摄入量数据使用Meng等[29]在广东省的调查数据(表2)。
表1 OPEs经口摄入参考剂量
Tab.1 Oral reference dose of OPEs
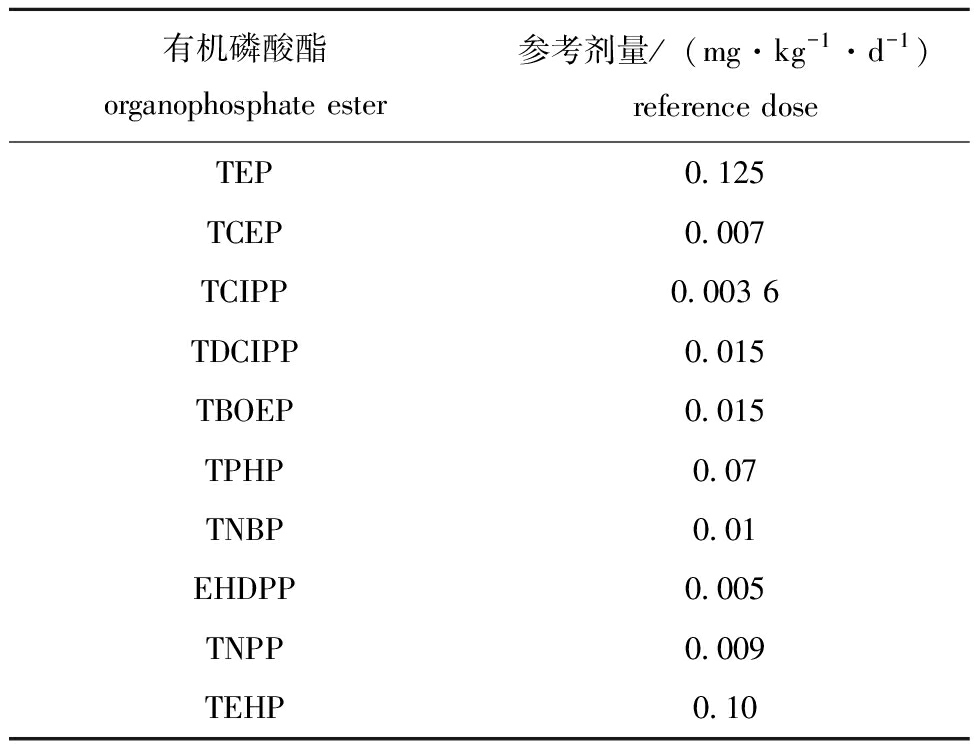
有机磷酸酯 organophosphate ester参考剂量/(mg·kg-1·d-1)reference doseTEPTCEPTCIPPTDCIPPTBOEPTPHPTNBPEHDPPTNPPTEHP0.1250.0070.003 60.0150.0150.070.010.0050.0090.10
表2 不同年龄段人群的鱼类摄入量[29]
Tab.2 Fish consumption of different age groups
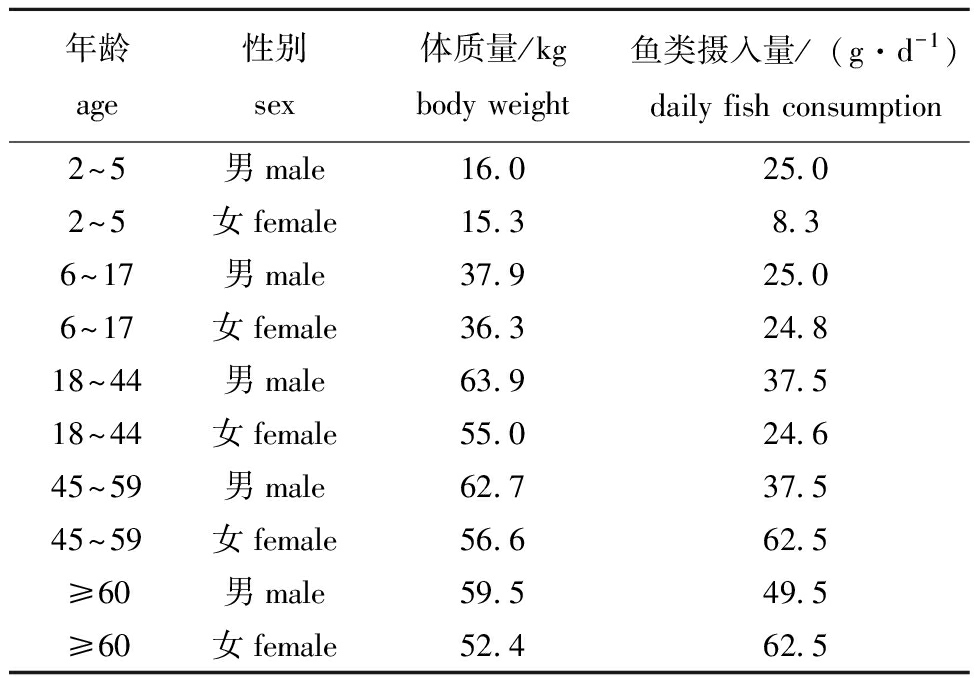
年龄age性别sex体质量/kgbody weight 鱼类摄入量/(g·d-1)daily fish consumption2~5男male16.025.02~5女female15.38.36~17男male37.925.06~17女female36.324.818~44男male63.937.518~44女female55.024.645~59男male62.737.545~59女female56.662.5≥60男male59.549.5≥60女female52.462.5
当IHI<1时,表示非致癌风险可接受,当IHI≥1时,表示有潜在非致癌风险[30]。
TCEP、TNBP、TEHP等OPEs化合物被美国环境保护署(US.EPA)列入致癌化合物清单,本研究水产品中TCEP、TNBP、TEHP 3种化合物的致癌风险采用CR(cancer risk)[29]模型评估,其计算公式为
(3)
式中:ICR为致癌风险;Ci、IDC、WBW含义和取值同公式(2)中一致;fEF(exposure frequency)为暴露频率(365 d/a);tED(exposure duration)为暴露持续时间(a);ICF(conversion factor)为单位转换因子(1×10-6 kg/mg);tAT(average time)为平均暴露时间(22 550 d);ISFO(oral cancer slope factor)为致癌性化合物经口摄入的致癌斜率因子[kg/(d·mg)],TCEP、TNBP、TEHP的ISFO数据采用US.EPA发布的数值[31],分别为0.02、0.009、0.003 2 kg/(d·mg)。
当ICR<1×10-6时,致癌风险可忽略;当1×10-6≤ICR≤1×10-4时,有潜在致癌风险;当ICR>1×10-4时,存在较高的潜在致癌风险[8]。
1.3 数据处理
试验数据采用SPSS 24.0软件进行统计分析。通过Shapiroe-Wilk检验数据是否符合正态分布。当数据为正态分布时,使用t检验分析数据之间差异性;否则,使用非参数检验。置信水平为95%,显著性水平设为0.05。
2 结果与分析
2.1 水产品中OPEs的含量
从表3可见:红海湾养殖水产品中,∑10OPEs含量范围为1.20~177.00 ng/g(干质量),平均值为(55.8±24.2)ng/g(干质量),OPEs在养殖水产品中的含量由高到低依次为青石斑鱼>金头鲷>尖吻鲈>星点笛鲷>花尾胡椒鲷>红鳍笛鲷>金鲳;捕捞水产品中,∑10OPEs含量范围为2.26~113.00 ng/g(干质量),平均值为(20.5±20.6)ng/g(干质量),显著低于养殖水产品中OPEs的含量(P<0.05);捕捞水产品中,底层或底栖品种内的OPEs含量较高,其中,龙头鱼中的∑10OPEs含量最高,其次为海鳗、三疣梭子蟹和红娘鱼,而中上层鱼类马鲛、鲐、黄姑鱼中的∑10OPEs含量较低,且底层或底栖水产品中的∑10OPEs含量显著高于中上层水产品中的∑10OPEs含量(P<0.05)。
表3 红海湾海域水产品中∑10OPEs含量
Tab.3 Concentration of ∑10OPEs in fishery products of Honghai Bay
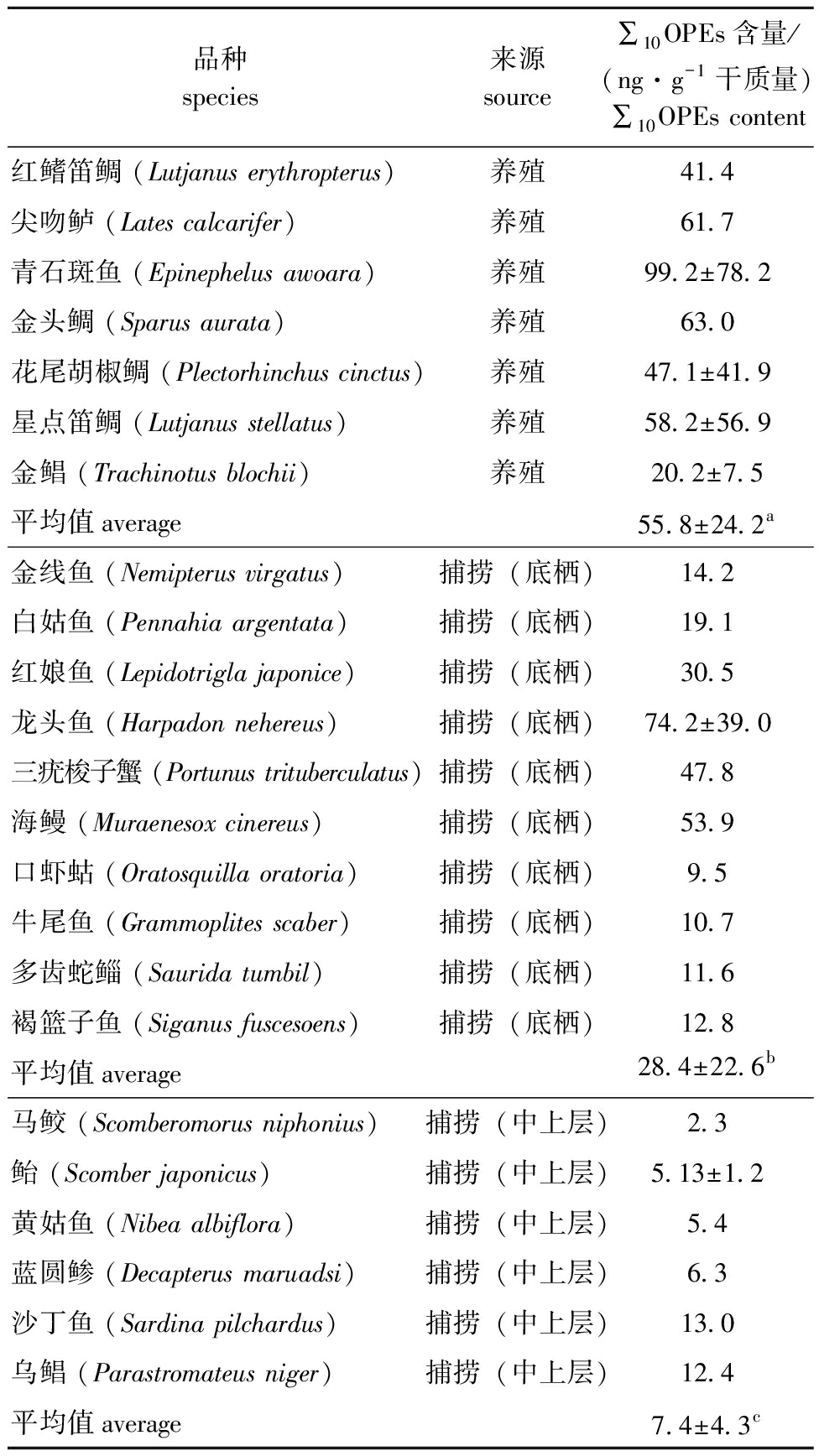
品种species来源source∑10OPEs含量/(ng·g-1干质量)∑10OPEs content红鳍笛鲷 (Lutjanus erythropterus)养殖41.4尖吻鲈 (Lates calcarifer)养殖61.7青石斑鱼 (Epinephelus awoara)养殖99.2±78.2金头鲷 (Sparus aurata)养殖63.0花尾胡椒鲷 (Plectorhinchus cinctus)养殖47.1±41.9星点笛鲷 (Lutjanus stellatus)养殖58.2±56.9金鲳 (Trachinotus blochii)养殖20.2±7.5平均值average55.8±24.2a金线鱼 (Nemipterus virgatus)捕捞(底栖)14.2白姑鱼 (Pennahia argentata)捕捞(底栖)19.1红娘鱼 (Lepidotrigla japonice)捕捞(底栖)30.5龙头鱼 (Harpadon nehereus)捕捞(底栖)74.2±39.0三疣梭子蟹 (Portunus trituberculatus)捕捞(底栖)47.8海鳗 (Muraenesox cinereus)捕捞(底栖)53.9口虾蛄 (Oratosquilla oratoria)捕捞(底栖)9.5牛尾鱼 (Grammoplites scaber)捕捞(底栖)10.7多齿蛇鲻 (Saurida tumbil)捕捞(底栖)11.6褐篮子鱼 (Siganus fuscesoens)捕捞(底栖)12.8平均值average28.4±22.6b马鲛 (Scomberomorus niphonius)捕捞(中上层)2.3鲐 (Scomber japonicus)捕捞(中上层)5.13±1.2黄姑鱼 (Nibea albiflora)捕捞(中上层)5.4蓝圆鲹 (Decapterus maruadsi)捕捞(中上层)6.3沙丁鱼 (Sardina pilchardus)捕捞(中上层)13.0乌鲳 (Parastromateus niger)捕捞(中上层)12.4平均值average7.4±4.3c
注:同列中标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05)。
Note:The means with different letters within the same column are significantly different in the groups at the 0.05 probability level,and the means with the same letter within the same column are not significant differences.
2.2 水产品中OPEs的组成
总体而言,TBOEP、TNBP、TCIPP、TCEP、TPHP 5种OPEs在红海湾海域养殖与捕捞水产品中的检出率最高,依次为96.7%、93.3%、86.7%、76.7%和66.7%,TEHP、EHDPP、TEP和TNPP次之,检出率为3.33%~30.0%,TDCIPP在所有样品中均未检出。
具体而言,养殖水产品中TCEP含量占比最高,占∑10OPEs的37.8%~81.8%;TBOEP和TCIPP次之,含量分别占∑10OPEs的12.4%~28.5%和4.96%~13.3%;TPHP在金鲳、花尾胡椒鲷、红鳍笛鲷中的含量占∑10OPEs的5.9%~16.3%,在其他品种中的含量占比为1%或以下;除金鲳(2.96%)外,TNBP在其余养殖品种中的含量占∑10OPEs的比值均小于0.7%(图1)。TCEP、TBOEP、TCIPP、TPHP 4种OPEs的总含量在∑10OPEs中的占比超过95%,是红海湾海域养殖水产品中最主要的OPEs污染物。
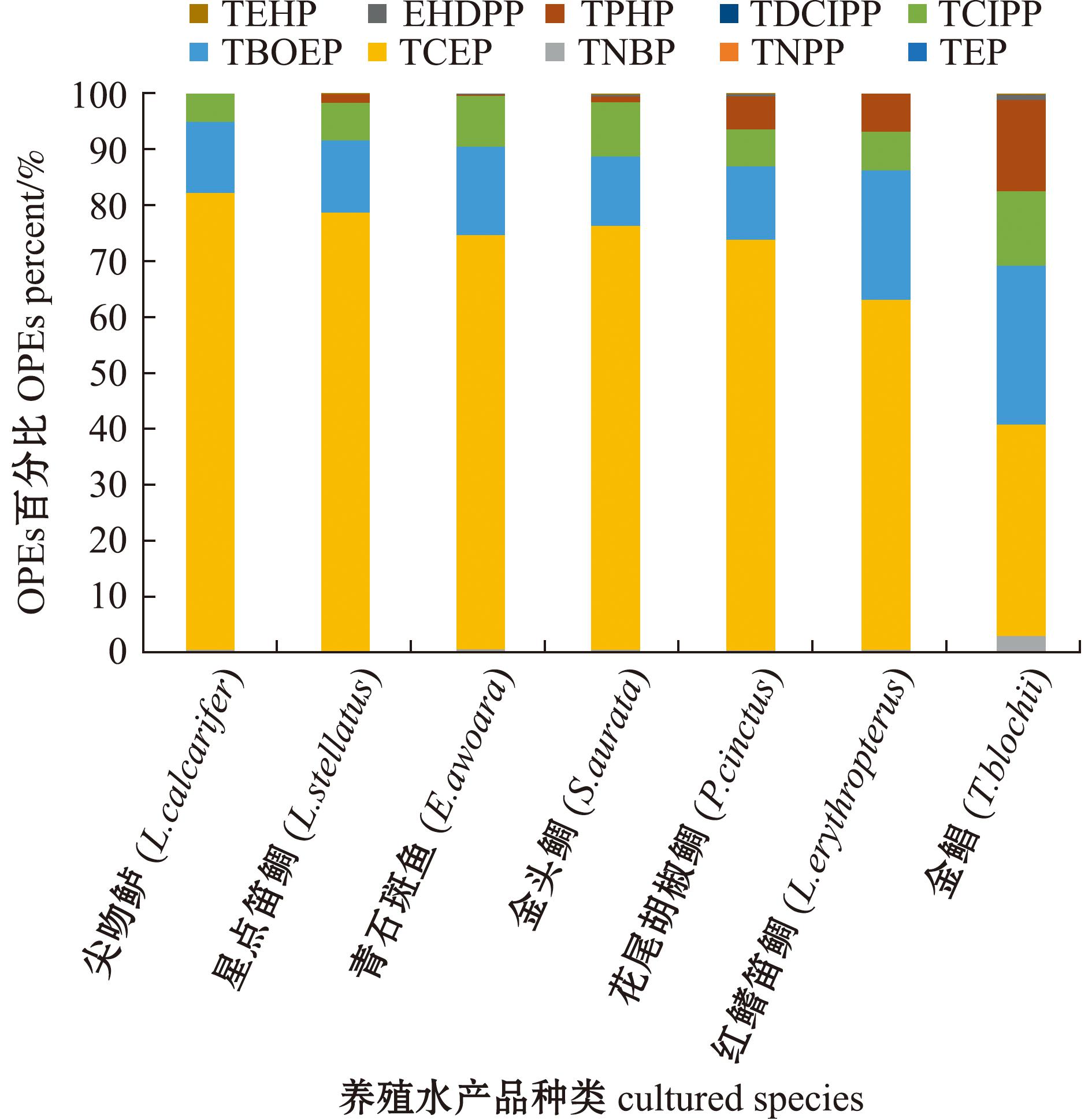
图1 红海湾海域养殖水产品中各OPE的百分比
Fig.1 Prercent of each OPE in cultured fishery products from Honghai Bay
捕捞水产品中,TCEP在三疣梭子蟹、海鳗和龙头鱼等(除口虾姑外)底栖或底层鱼类中的含量占∑10OPEs的30.2%~72.9%,是最主要的OPEs污染物,其次为TBOEP和TCIPP,含量分别占∑10OPEs的15.1%~42.2%和6.98%~21.2%,TNBP含量占∑10OPEs的1.27%~3.29%(除海鳗、三疣梭子蟹外)(图2)。中上层鱼类中,TBOEP是主要的污染物,含量占∑10OPEs的40.1%~89.0%,其次为TCIPP,含量占∑10OPEs的10.6%~35.4%(除马鲛外),TPHP在蓝圆鲹、鲐和黄姑鱼样品中的含量仅次于TCIPP,含量分别占∑10OPEs的8.54%、22.9%和14.9%;捕捞水产品中,TNBP化合物也存在较高的污染浓度,其含量占∑10OPEs的4.76%~11.0%(图2)。
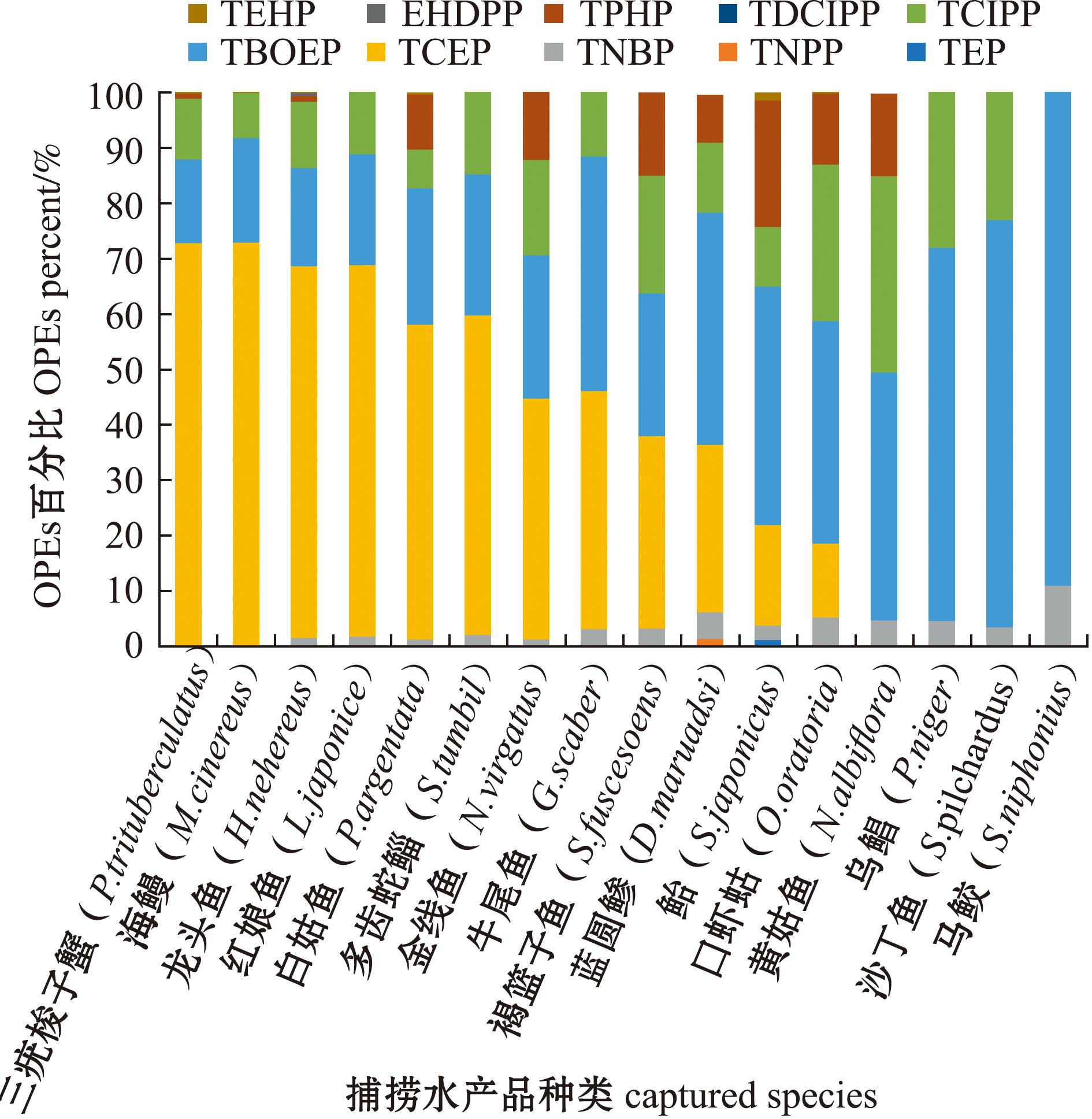
图2 红海湾海域捕捞水产品中各OPE的百分比
Fig.2 Percent of each OPE in captured fishery products from Honghai Bay
由此可见,TBOEP、TCIPP、TCEP、TNBP、TPHP几类化合物在红海湾海域水产品中不仅具有较高检出频率,而且是水产品中含量较高的污染物。
2.3 OPEs暴露水平和健康风险
2.3.1 OPEs暴露水平 从表4可见,不同年龄组和性别人群对10种OPEs的每日估计摄入量范围为0.73~19.83 ng/(kg·d),其中,居民通过膳食养殖水产品摄入OPEs的量为5.68~19.83 ng/(kg·d),显著高于通过膳食捕捞水产品摄入OPEs的量[0.73~8.59 ng/(kg·d)](P<0.05),而通过膳食捕捞底栖水产品摄入OPEs的量[2.46~8.59 ng/(kg·d)]显著高于通过膳食捕捞中上层水产品摄入OPEs的量[0.73~2.54 ng/(kg·d)](P<0.05)。5个年龄组男性和女性中,2~5岁年龄组男性对OPEs的每日估计摄入量最高,其次分别为60岁以上年龄组女性、45~59岁年龄组女性和60岁以上年龄组男性,18~44岁女性对OPEs的每日估计摄入量最低。
表4 不同来源水产品中OPEs每日估计摄入量、危害指数和致癌风险评价结果
Tab.4 Estimated daily intake,hazard index and cancer risk of OPEs by fishery products from different sources
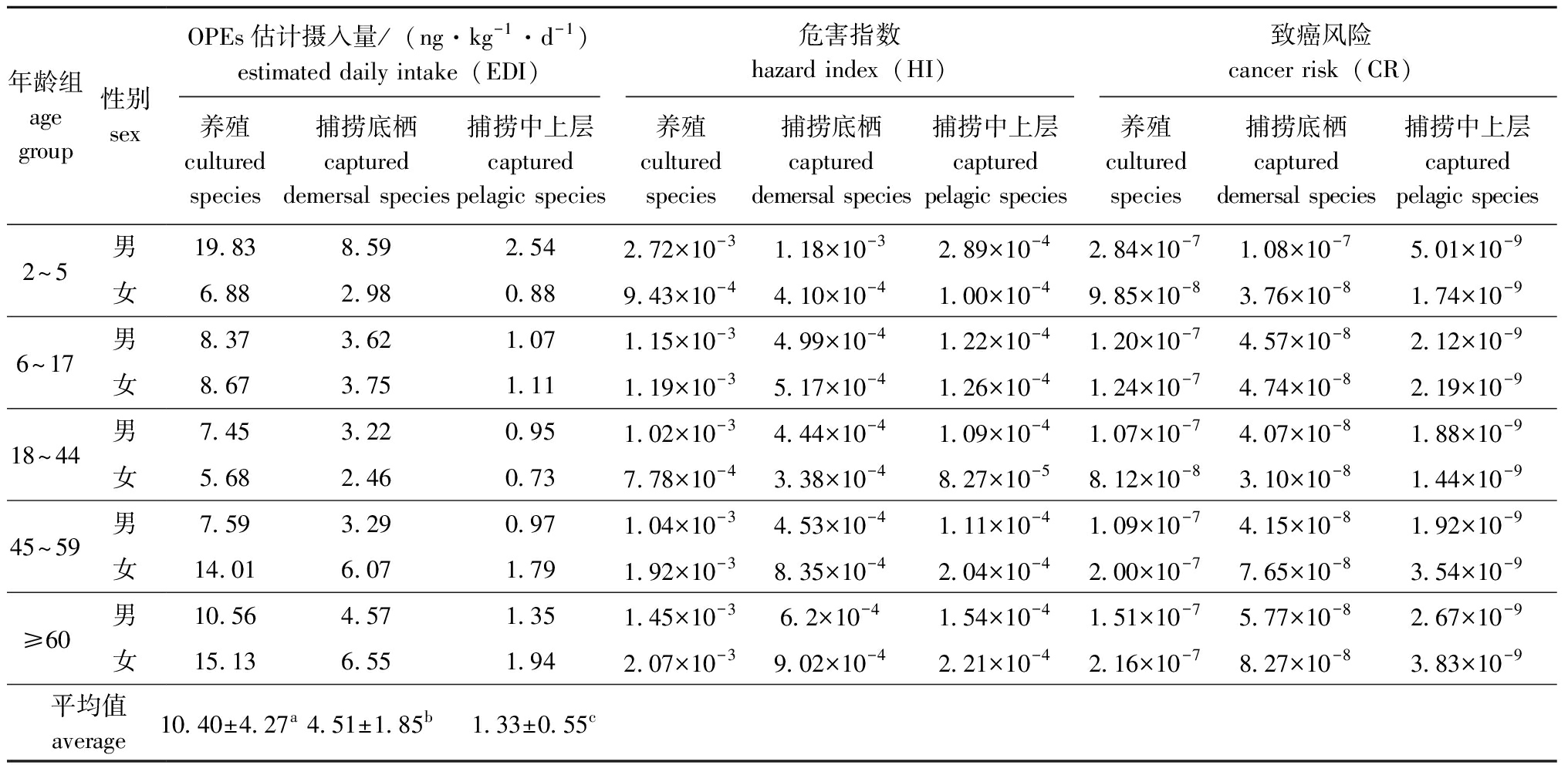
年龄组age group性别sexOPEs估计摄入量/(ng·kg-1 ·d-1)estimated daily intake(EDI)危害指数hazard index(HI)致癌风险cancer risk(CR)养殖cultured species捕捞底栖captured demersal species捕捞中上层captured pelagic species养殖cultured species捕捞底栖captured demersal species捕捞中上层captured pelagic species养殖cultured species捕捞底栖captured demersal species捕捞中上层captured pelagic species2~5男 19.838.592.542.72×10-31.18×10-32.89×10-42.84×10-71.08×10-75.01×10-9女6.882.980.889.43×10-44.10×10-41.00×10-49.85×10-83.76×10-81.74×10-96~17男 8.373.621.071.15×10-34.99×10-41.22×10-41.20×10-74.57×10-82.12×10-9女8.673.751.111.19×10-35.17×10-41.26×10-41.24×10-74.74×10-82.19×10-918~44男 7.453.220.951.02×10-34.44×10-41.09×10-41.07×10-74.07×10-81.88×10-9女5.682.460.737.78×10-43.38×10-48.27×10-58.12×10-83.10×10-81.44×10-945~59男 7.593.290.971.04×10-34.53×10-41.11×10-41.09×10-74.15×10-81.92×10-9女14.016.071.791.92×10-38.35×10-42.04×10-42.00×10-77.65×10-83.54×10-9≥60男 10.564.571.351.45×10-36.2×10-41.54×10-41.51×10-75.77×10-82.67×10-9女15.136.551.942.07×10-39.02×10-42.21×10-42.16×10-78.27×10-83.83×10-9平均值average10.40±4.27a4.51±1.85b1.33±0.55c
注:同行中标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05)。
Note:The means with different letters within the same line are significantly different in the groups at the 0.05 probability level,and the means with the same letter within the same line are not significant differences.
2.3.2 OPEs健康风险 OPEs非致癌风险评估结果显示,红海湾海域水产品中10种OPEs残留引起的危害指数HI为8.27×10-5~2.7×10-3(表4),低于潜在非致癌风险阈值(IHI≥1)3~5个数量级,表明目前红海湾海域水产品中OPEs污染对人体产生的非致癌健康风险可接受。
致癌风险评估结果显示,红海湾海域水产品中残留的TCEP、TNBP、TEHP 3种致癌物的总致癌风险ICR值为1.44×10-9~2.84×10-7(表4),低于潜在致癌风险阈值(1×10-6≤ICR≤1×10-4),表明致癌风险可忽略。
3 讨论
3.1 水产品中OPEs含量特征
OPEs在水产品中的污染水平受OPEs环境污染水平、OPEs理化性质和水产品栖息习性等因素共同影响。本研究中,红海湾海域养殖水产品OPEs含量与北部湾养殖水产品中接近[32],高于同水域和其他水域的捕捞水产品中OPEs含量(表5)。虽然不同水域捕捞水产品中OPEs含量存在差异,但均表现出与红海湾海域相似的含量分布趋势,即底层水产品中OPEs含量高于中上层水产品。Brandsma等[1]在荷兰Western Scheldt河口食物网生物中研究发现,底栖水生生物中OPEs含量高于中上层水生生物。Bekele等[20]在莱州湾的研究中也发现类似现象,即底层鱼类OPEs含量高于中上层鱼类。水产品中OPEs污染水平产生差异的原因可能与生物栖息习性及栖息环境中OPEs污染水平密切相关。
表5 不同水域水生生物中OPEs污染含量和组成比较
Tab.5 Comparison of concentration and composition of OPEs in aquatic organisms from different sampling sites
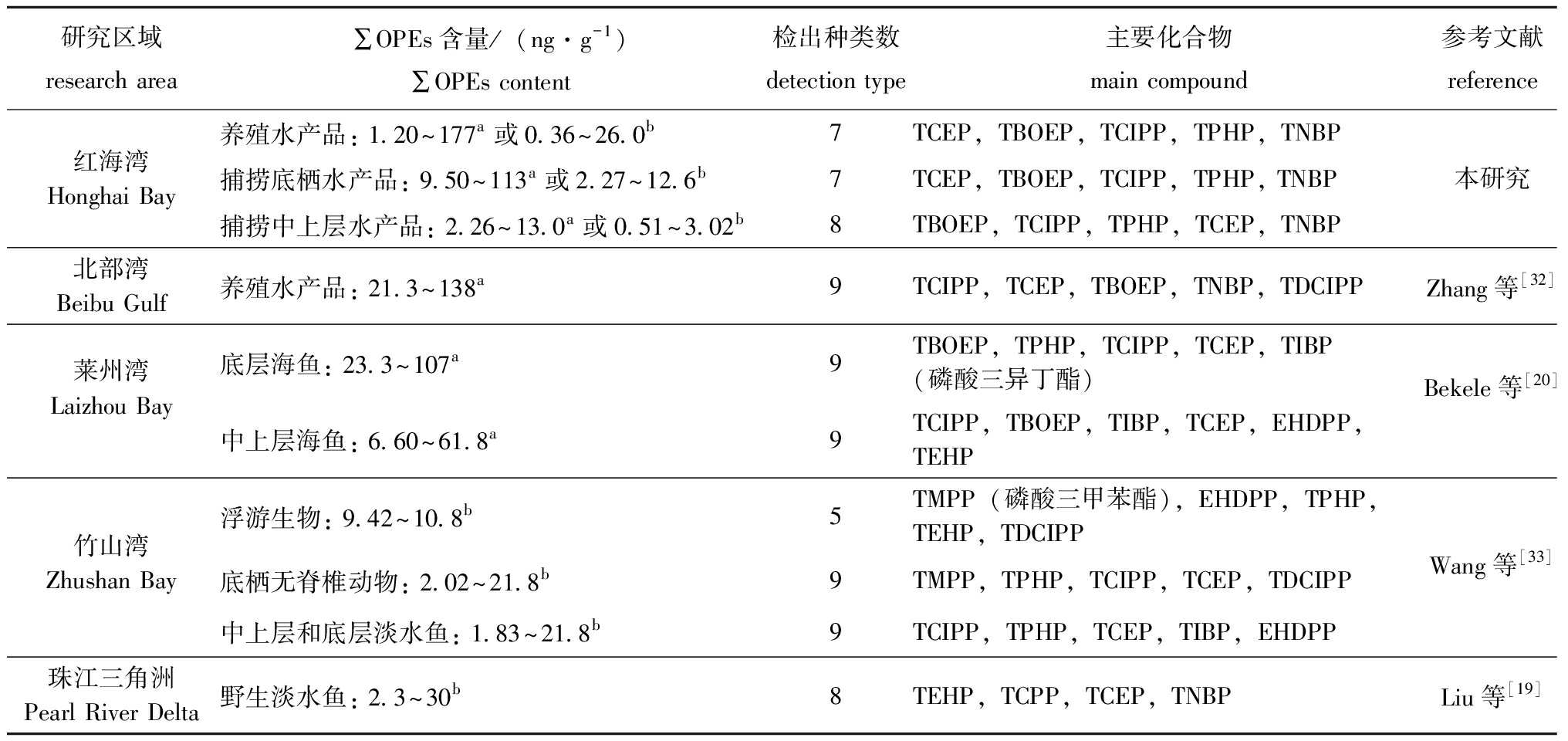
研究区域research area∑OPEs含量/(ng·g-1)∑OPEs content 检出种类数detection type主要化合物main compound参考文献reference养殖水产品: 1.20~177a或0.36~26.0b7TCEP,TBOEP,TCIPP,TPHP,TNBP红海湾Honghai Bay捕捞底栖水产品: 9.50~113a或2.27~12.6b7TCEP,TBOEP,TCIPP,TPHP, TNBP本研究 捕捞中上层水产品: 2.26~13.0a或0.51~3.02b8TBOEP,TCIPP,TPHP,TCEP,TNBP北部湾 Beibu Gulf养殖水产品: 21.3~138a9TCIPP,TCEP,TBOEP,TNBP,TDCIPPZhang等[32]莱州湾Laizhou Bay底层海鱼: 23.3~107 a9TBOEP,TPHP,TCIPP,TCEP,TIBP(磷酸三异丁酯)Bekele等[20]中上层海鱼: 6.60~61.8a9TCIPP,TBOEP,TIBP,TCEP,EHDPP,TEHP浮游生物: 9.42~10.8b5TMPP(磷酸三甲苯酯),EHDPP,TPHP,TEHP,TDCIPP竹山湾Zhushan Bay底栖无脊椎动物: 2.02~21.8b9TMPP,TPHP,TCIPP,TCEP,TDCIPPWang等[33]中上层和底层淡水鱼: 1.83~21.8b9TCIPP,TPHP,TCEP,TIBP,EHDPP珠江三角洲Pearl River Delta野生淡水鱼: 2.3~30b8TEHP,TCPP,TCEP,TNBPLiu等[19]
注:a表示OPEs浓度以干质量计;b表示OPEs浓度以湿质量计。
Note:a and b indicate OPEs concentrations calculated based on dry tissue mass and wet tissue mass,respectively.
3.2 水产品中OPEs组成特征
TCEP、TBOEP、TCIPP、TNBP、TPHP 5种化合物不仅是红海湾海域水产品中主要的检出污染物类型,还是太湖[33]、莱州湾[20]和珠江[19]等水域水生生物中的主要OPEs污染物。OPEs在水产品中的检出频率和污染浓度与其自身工业生产使用量密切相关。据估计,2020年中国TCIPP产量为44 681 t,TCEP为30 957 t,TBOEP为22 236 t,TPHP为14 493 t,TNBP为14 907 t[34]。由于工业上的大量生产和使用,导致这几种化合物普遍释放于水产养殖环境中,并通过生物呼吸、摄食摄入等途径富集于水产品内。在邻近红海湾的珠江三角洲水体中,含量较高的OPEs污染物依次为TBOEP、TCIPP、TCEP、TNBP,沉积物中主要为TCEP、TCIPP、TNBP、TPHP[35]。Zhang等[35]对南海北部湾养殖海水、沉积物及养殖水产品内OPEs污染进行分析,结果显示,海水、沉积物和养殖水产品中主要OPEs污染物组成基本一致,均以TCIPP、TCEP、TBOEP、TNBP化合物为主,污染物组成特征与本研究结果类似。
本研究中,针对不同栖息习性的水生生物分析结果显示,红海湾海域底层或底栖捕捞水产品中主要OPEs污染物与养殖水产品中接近,TCEP、TBOEP、TCIPP 3种OPEs污染浓度最高,而中上层捕捞水产品中高水平污染物依次为TBOEP、TCIPP、TPHP、TNBP,与养殖水产品和底层捕捞水产品存在一定差异,这种差异可能与水生生物栖息习性有关。近岸网箱养殖鱼类的养殖位置相对固定,底层或底栖鱼类洄游性不强,栖息地相对固定,如多齿蛇鲻(Saurida tumbil)、白姑鱼(Pennahia argentata)无明显长距离洄游,主要做水域深浅移动,底栖或底层鱼类持续、稳定暴露于污染物中[36],因此,TCEP、TBOEP、TCIPP 3种主要污染物在养殖鱼类和底栖鱼类中含量比例非常接近;而中上层鱼类栖息习性则与之不同,分布水层高、游泳速度快、洄游范围广,且有较明显的远距离生殖洄游或大尺度昼夜垂直移动的特点,如乌鲳(Parastromateus niger)、马鲛、沙丁鱼(Sardina pilchardus)等,迁移过程中环境污染物含量水平是变化的,所以富集的污染物组成与底层或养殖鱼类不同[36]。
3.3 水产品OPEs摄入风险
对OPEs生态毒理学研究已证实,OPEs对生物体具有致癌风险与非致癌风险(神经发育毒性,致激素分泌失调等)。本研究中,通过计算不同年龄段男性和女性每日膳食水产品摄入OPEs的含量,评估可能导致的非致癌风险和致癌风险水平,研究结果显示,低龄男性和高龄女性表现出相对较高的暴露水平和风险,意味着这两个群体更易受到OPEs的威胁,应高度关注。总的来看,现阶段红海湾海域养殖和捕捞水产品中OPEs对居民造成的致癌与非致癌健康风险均在可接受阈值内,该海域水产品相对安全。
4 结论
1)红海湾海域养殖和捕捞水产品中均检出OPEs残留,OPEs含量在养殖水产品内显著高于捕捞水产品,在捕捞底栖水产品中的含量显著高于捕捞中上层水产品。
2)TBOEP、TCEP 、TCIPP、 TNBP、TPHP 5种工业中使用较多的OPEs化合物是红海湾海域水产品中的主要污染物类型。
3)暴露评估显示,现阶段红海湾海域水产品中OPEs残留引起的人体健康风险尚可接受。
综上,OPEs已普遍存在于红海湾海域水产品中,但健康风险可接受。然而,随着OPEs的工业使用量持续快速增长,且红海湾毗邻的珠江口和粤东地区制造业发达,OPEs的水产品污染风险也将持续增高,因此,红海湾海域水产品中OPEs污染仍需进一步关注。
[1] BRANDSMA S H,LEONARDS P E G,LESLIE H A,et al.Tracing organophosphorus and brominated flame retardants and plasticizers in an estuarine food web[J].The Science of the Total Environment,2015,505:22-31.
[2] BEKELE T G,ZHAO H X,YANG J,et al.A review of environmental occurrence,analysis,bioaccumulation,and toxicity of organophosphate esters[J].Environmental Science and Pollution Research International,2021,28(36):49507-49528.
[3] GUO J H,VENIER M,SALAMOVA A,et al.Bioaccumulation of dechloranes,organophosphate esters,and other flame retardants in Great Lakes fish[J].The Science of the Total Environment,2017,583:1-9.
[4] SCHMIDT N,CASTRO-JIMENEZ J,OURSEL B,et al.Phthalates and organophosphate esters in surface water,sediments and zooplankton of the NW Mediterranean Sea:exploring links with microplastic abundance and accumulation in the marine food web[J].Environmental Pollution,2021,272:115970.
[5] LAI N L S,KWOK K Y,WANG X H,et al.Assessment of organophosphorus flame retardants and plasticizers in aquatic environments of China (Pearl River Delta,South China Sea,Yellow River Estuary) and Japan (Tokyo Bay)[J].Journal of Hazardous Materials,2019,371:288-294.
[6] WANG X L,ZHU L Y,ZHONG W J,et al.Partition and source identification of organophosphate esters in the water and sediment of Taihu Lake,China[J].Journal of Hazardous Materials,2018,360:43-50.
[7] FANG L D,LIU A F,ZHENG M G,et al.Occurrence and distribution of organophosphate flame retardants in seawater and sediment from coastal areas of the East China and Yellow Seas[J].Environmental Pollution,2022,302:119017.
[8] XING L Q,TAO M,ZHANG Q,et al.Occurrence,spatial distribution and risk assessment of organophosphate esters in surface water from the Lower Yangtze River Basin[J].The Science of the Total Environment,2020,734:139380.
[9] SHI Y F,ZHANG Y,DU Y M,et al.Occurrence,composition and biological risk of organophosphate esters (OPEs) in water of the Pearl River Estuary,south China[J].Environmental Science and Pollution Research,2020,27(13):14852-14862.
[10] CHEN M Q,GAN Z W,QU B,et al.Temporal and seasonal variation and ecological risk evaluation of flame retardants in seawater and sediments from Bohai Bay near Tianjin,China during 2014 to 2017[J].Marine Pollution Bulletin,2019,146:874-883.
[11] LI R W,WANG H Q,MI C,et al.The adverse effect of TCIPP and TCEP on neurodevelopment of zebrafish embryos/larvae[J].Chemosphere,2019,220:811-817.
[12] WANG Q W,LAI N L S,WANG X F,et al.Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae[J].Environmental Science &Technology,2015,49(8):5123-5132.
[13] 王思敏,李学彦,周启星,等.三(1,3-二氯-2-丙基)磷酸酯对大鼠的神经毒性效应[J].生态毒理学报,2019,14(3):186-195.
WANG S M,LI X Y,ZHOU Q X,et al.Tris(1,3-dichloro-2-propyl)phosphate(TDCPP) induced neurotoxic effects in rats[J].Asian Journal of Ecotoxicology,2019,14(3):186-195.(in Chinese)
[14] XIONG H,HUANG Y Y,MAO Y C,et al.Inhibition in growth and cardiotoxicity of tris (2-butoxyethyl) phosphate through down-regulating Wnt signaling pathway in early developmental stage of zebrafish (Danio rerio)[J].Ecotoxicology and Environmental Safety,2021,208:111431.
[15] KANDA K,ITO S,KOH D H,et al.Effects of tris(2-chloroethyl) phosphate exposure on chicken embryos in a shell-less incubation system[J].Ecotoxicology and Environmental Safety,2021,207:111263.
[16] AL-SALEM A M,SAQUIB Q,SIDDIQUI M A,et al.Organophosphorus flame retardant (tricresyl phosphate) trigger apoptosis in HepG2 cells:transcriptomic evidence on activation of human cancer pathways[J].Chemosphere,2019,237:124519.
[17] AL-SALEM A M,SAQUIB Q,SIDDIQUI M A,et al.Tris(2-chloroethyl) phosphate (TCEP) elicits hepatotoxicity by activating human cancer pathway genes in HepG2 cells[J].Toxics,2020,8(4):109.
[18] HOU R,LIU C,GAO X Z,et al.Accumulation and distribution of organophosphate flame retardants (PFRs) and their di-alkyl phosphates (DAPs) metabolites in different freshwater fish from locations around Beijing,China[J].Environmental Pollution,2017,229:548-556.
[19] LIU Y E,LUO X J,HUANG L Q,et al.Organophosphorus flame retardants in fish from rivers in the Pearl River Delta,south China[J].The Science of the Total Environment,2019,663:125-132.
[20] BEKELE T G,ZHAO H X,WANG Q Z.Tissue distribution and bioaccumulation of organophosphate esters in wild marine fish from Laizhou Bay,north China:implications of human exposure via fish consumption[J].Journal of Hazardous Materials,2021,401:123410.
[21] GIULIVO M,CAPRI E,KALOGIANNI E,et al.Occurrence of halogenated and organophosphate flame retardants in sediment and fish samples from three European River Basins[J].The Science of the Total Environment,2017,586:782-791.
[22] 赵永强,李娜,李来好,等.水产品质量与安全控制的蛋白质组学研究[J].大连海洋大学学报,2016,31(3):344-350.
ZHAO Y Q,LI N,LI L H,et al.Application of proteomics in regulation of aquatic products quality and safety:a review[J].Journal of Dalian Ocean University,2016,31(3):344-350.(in Chinese)
[23] HU Y X,SUN Y X,LI X,et al.Organophosphorus flame retardants in mangrove sediments from the Pearl River Estuary,south China[J].Chemosphere,2017,181:433-439.
[24] LIU Y E,LUO X J,CORELLA P,et al.Organophosphorus flame retardants in a typical freshwater food web:bioaccumulation factors,tissue distribution,and trophic transfer[J].Environmental Pollution,2019,255(Pt 2):113286.
[25] ZENG Y M,KE C L,LIU Q,et al.Simultaneous determination of organophosphate ester flame retardants in water and sediments by Gas Chromatography-Tandem Mass Spectrometry (GC-MS/MS)[J].Analytical Letters,2022,55(18):2928-2943.
[26] JIANG Y S,LIU Z B,WU D T,et al.Toxaphene levels in retail food from the Pearl River Delta area of south China and an assessment of dietary intake[J].Chemosphere,2016,152:318-327.
[27] United States Environmental Protection Agency (US. EPA).Human health risk assessment protocol for hazardous [EB/OL].2005 [2023-6-10].https://archive.epa.gov/epawaste/hazard/tsd/td/web/html/riskvol.html.
[28] LUO Q,WU Z P,WANG C C,et al.Seasonal variation,source identification,and risk assessment of organophosphate ester flame retardants and plasticizers in surficial sediments from Liao River Estuary wetland,China[J].Marine Pollution Bulletin,2021,173(Pt A):112947.
[29] MENG X Z,ZENG E Y,YU L P,et al.Assessment of human exposure to polybrominated diphenyl ethers in China via fish consumption and inhalation[J].Environmental Science &Technology,2007,41(14):4882-4887.
[30] XING L Q,ZHANG Q,SUN X,et al.Occurrence,distribution and risk assessment of organophosphate esters in surface water and sediment from a shallow freshwater lake,China[J].The Science of the Total Environment,2018,636:632-640.
[31] United States Environmental Protection Agency (US.EPA).Comptox chemicals dashboard [EB/OL].2020[2023-6-10].https://comptox.epa.gov/dashboard/.
[32] ZHANG R J,YU K F,LI A,et al.Occurrence,phase distribution,and bioaccumulation of organophosphate esters (OPEs) in mariculture farms of the Beibu Gulf,China:a health risk assessment through seafood consumption[J].Environmental Pollution,2020,263(Pt B):114426.
[33] WANG X L,ZHONG W J,XIAO B W,et al.Bioavailability and biomagnification of organophosphate esters in the food web of Taihu Lake,China:impacts of chemical properties and metabolism[J].Environment International,2019,125:25-32.
[34] HUANG J N,YE L J,FANG M L,et al.Industrial production of organophosphate flame retardants (OPFRs):big knowledge gaps need to be filled?[J].Bulletin of Environmental Contamination and Toxicology,2022,108(5):809-818.
[35] ZHANG Y,ZHENG X B,WEI L F,et al.The distribution and accumulation of phosphate flame retardants (PFRs) in water environment[J].The Science of the Total Environment,2018,630:164-170.
[36] 贾晓平,李永松,李纯厚,等.南海专属经济区和大陆架渔业生态环境和渔业资源[M].北京:科学出版社,2004:303-542.
JIA X P,LI Y S,LI C H,et al.Fisheries ecosystem and fisheries resources in the exclusive economic zone and continental shelf of the South China Sea [M].Beijing: China Science and Technology Press,2004:303-542.(in Chinese)