微管(microtubule)是真核生物细胞骨架的主要成分,参与保持细胞形态、运动、分裂,以及胞内物质运输和分泌等重要的生物学过程[1]。近年研究显示,微管结构与逆境应答也有密切关系[2-3]。α-微管蛋白(α-Tubulin)作为微管主要成分之一,在生物生长过程中的调控作用受到关注[4]。研究表明,细胞骨架可能是水生生物低温损伤的重要位点之一[5-6]。海参的再生功能也与α-Tubulin关系密切[7]。目前,已经鉴定了7种微管蛋白,包括微管蛋白α、β、γ、δ、ε、ζ和η [8-9],微管蛋白由多基因编码,不同微管蛋白的功能还有待进一步研究[10-12]。
马氏珠母贝(Pinctada fucata martensii)属暖水性贝类,低温耐受能力弱,冬季寒潮导致的大规模死亡对马氏珠母贝海水珍珠养殖产业造成了严重影响。其中,2008年1—2月受特大寒潮影响,广东省湛江流沙湾70%的珍珠贝死亡(据湛江市珍珠协会资料,未发表)。因此,水温是马氏珠母贝养殖区域的重要限制性因子,培育马氏珠母贝耐低温品系是开展珍珠贝北移养殖及扩展珍珠贝养殖区域的前提。本课题组自2013年起致力于培育马氏珠母贝耐低温选育系,目前已经选育至F3代,通过对耐低温选育系F1和对照群体的转录组分析发现,马氏珠母贝α-Tubulin基因在耐低温品系中表达上调[13],说明该基因可作为马氏珠母贝耐低温的候选基因。筛选与抗低温相关的DNA分子标记应用于分子辅助育种,可加速抗寒性遗传改良进程。单核苷酸多态性(single nucleotide polymorphism,SNP)作为遗传育种研究的首选标记之一,具有多态性高、分布广泛及遗传稳定性与准确性高的优点[14-15]。因此,对α-Tubulin进行SNP多态性分析,探究其与耐低温能力的关系,有助于进一步培育马氏珠母贝耐低温品系。本研究中,通过RACE克隆技术获得马氏珠母贝α-Tubulin基因,对其进行了生物信息学分析,同时分析了其组织表达模式、低温胁迫下鳃组织的时序表达模式及SNP位点,以期为开展贝类对不良环境的适应性研究提供基础数据。
1 材料与方法
1.1 材料
试验用马氏珠母贝活力较强,壳长为69.23 mm±3.80 mm,采自广东省雷州市后洪村海区。
1.2 方法
1.2.1 试验设计及样品采集 随机取8只试验贝的全组织,经液氮速冻后于-80 ℃超低温冰箱中保存备用。其余试验贝在室温中适应2 d,清除死贝及明显不健康的贝后,将150只试验贝随机平分为3组。3组试验贝分别用锥形笼吊于3个300 L的养殖桶养殖。采用控温系统控制水温,设置12、17 ℃低温组和22 ℃对照组。养殖过程中每天投喂等量的单胞藻。在控温开始后6、24、72、120 h时,从每组随机取8只贝剪取鳃,经液氮速冻后于-80 ℃超低温冰箱中保存备用。
1.2.2 α-Tubulin基因的克隆 利用巢式PCR扩增获得α-Tubulin的3′和5′序列。PCR目的产物经连接后转化至DH5α中,用氨苄选择培养基培养,挑取阳性菌测序。将α-Tubulin 3′和5′测序结果与马氏珠母贝基因组中的α-Tubulin序列[16]拼接得到全长序列。用Primer Primer 5.0软件设计巢式PCR扩增引物及荧光定量PCR引物(表1)。
表1 引物序列
Tab.1 Primer sequences
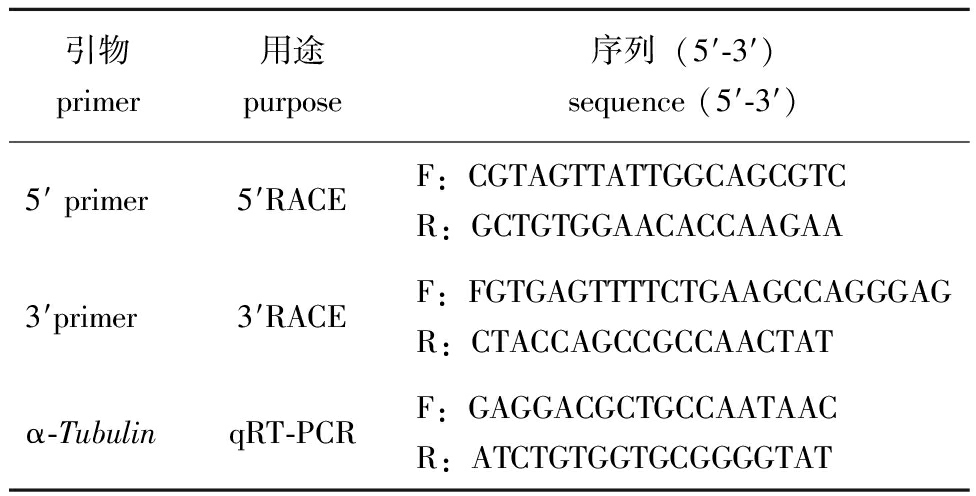
引物 primer用途 purpose序列(5′-3′) sequence (5′-3′)5′ primer5′RACEF:CGTAGTTATTGGCAGCGTCR:GCTGTGGAACACCAAGAA3′primer3′RACEF:FGTGAGTTTTCTGAAGCCAGGGAGR:CTACCAGCCGCCAACTATα-TubulinqRT-PCRF:GAGGACGCTGCCAATAACR:ATCTGTGGTGCGGGGTAT
1.2.3 生物信息学分析 采用DNAMAN推导α-Tubulin编码的氨基酸序列;分别使用ORF Finder、ExPASy-ProtParam tool、SignalP 4.1及TMHMM Server软件预测α-Tubulin的开放阅读框、氨基酸理化性质、信号肽及跨膜结构。
1.2.4 α-Tubulin基因的SNP筛选 用于SNP筛选的马氏珠母贝来自第三代耐低温品系[17],2018年10月,从耐低温选育系(R)F3和北部湾野生群体(W)中分别随机取30个个体,剪取其闭壳肌装于冻存管,用液氮速冻后置于-80 ℃超低温冰箱中备用。提取R和W群体闭壳肌的DNA,通过重测序用于α-Tubulin基因的SNP筛选。
1.2.5 SNP位点的遗传多态性及低温耐受性分析
采用Popgene 32软件计算等位基因数、期望杂合度(He)、等位基因频率、有效等位基因数(Ne)、哈迪-温伯格平衡(HWE)及观测杂合度(Ho);采用PIC_CALC软件计算SNP位点的多态信息含量(PIC)。采用SPSS 22.0软件进行卡方检验,对SNP位点与马氏珠母贝的低温耐受性状做关联分析;采用Haploview 4.2软件计算R2和D′值,并进行连锁不平衡和单倍型分析。
1.3 数据处理
以β-actin为内参基因,采用2-ΔΔCt法计算α-Tubulin的相对表达量。采用SPSS 22.0软件进行单因素方差分析,用Tukey法进行组间多重比较,显著性水平设为0.05。
2 结果与分析
2.1 α-Tubulin序列
通过RACE技术获得的α-Tubulin全长序列长度为2 107 bp,5′ UTR为75 bp,3′ UTR为667 bp,开放阅读框(ORF)为1 365 bp,编码454个氨基酸(图1)。预测α-Tubulin蛋白无信号肽、无跨膜结构,具有典型的Tubulin和Tubulin-C结构域。

5′和3′非编码区用小写字母表示;开放阅读框及推导的氨基酸序列用大写字母表示;黑色下划线和蓝色下划线部分分别为保守结构域Tubulin和Tubulin-C。
5′ UTR and 3′ UTR are indicated with small letters;open reading frame and the deduced amino acid sequences are indicated with capital letters;the underlined part which in black and blue are respectively the conservative domains Tubulin and Tubulin-C.
图1 马氏珠母贝α-Tubulin核苷酸序列及推导的氨基酸序列
Fig.1 Nucleotide sequence and the deduced amino acid sequence of α-Tubulin in Pinctada fucata martensii
2.2 α-Tubulin氨基酸序列进化树的构建
将马氏珠母贝α-Tubulin氨基酸序列与青鳉(Oryzias latipes)等15个物种的α-Tubulin氨基酸序列构建系统进化树,结果显示,马氏珠母贝与太平洋牡蛎(Crassostrea gigas)聚为一支,说明二者亲缘关系较近(图2)。

图2 α-Tubulin系统发育树
Fig.2 Phylogenetic trees of α-Tubulin
2.3 α-Tubulin基因的组织表达
从图3可见,α-Tubulin基因在马氏珠母贝各组织中均有表达,在足中表达量最高(P<0.05),其次是鳃,闭壳肌中表达量最低(P<0.05)。
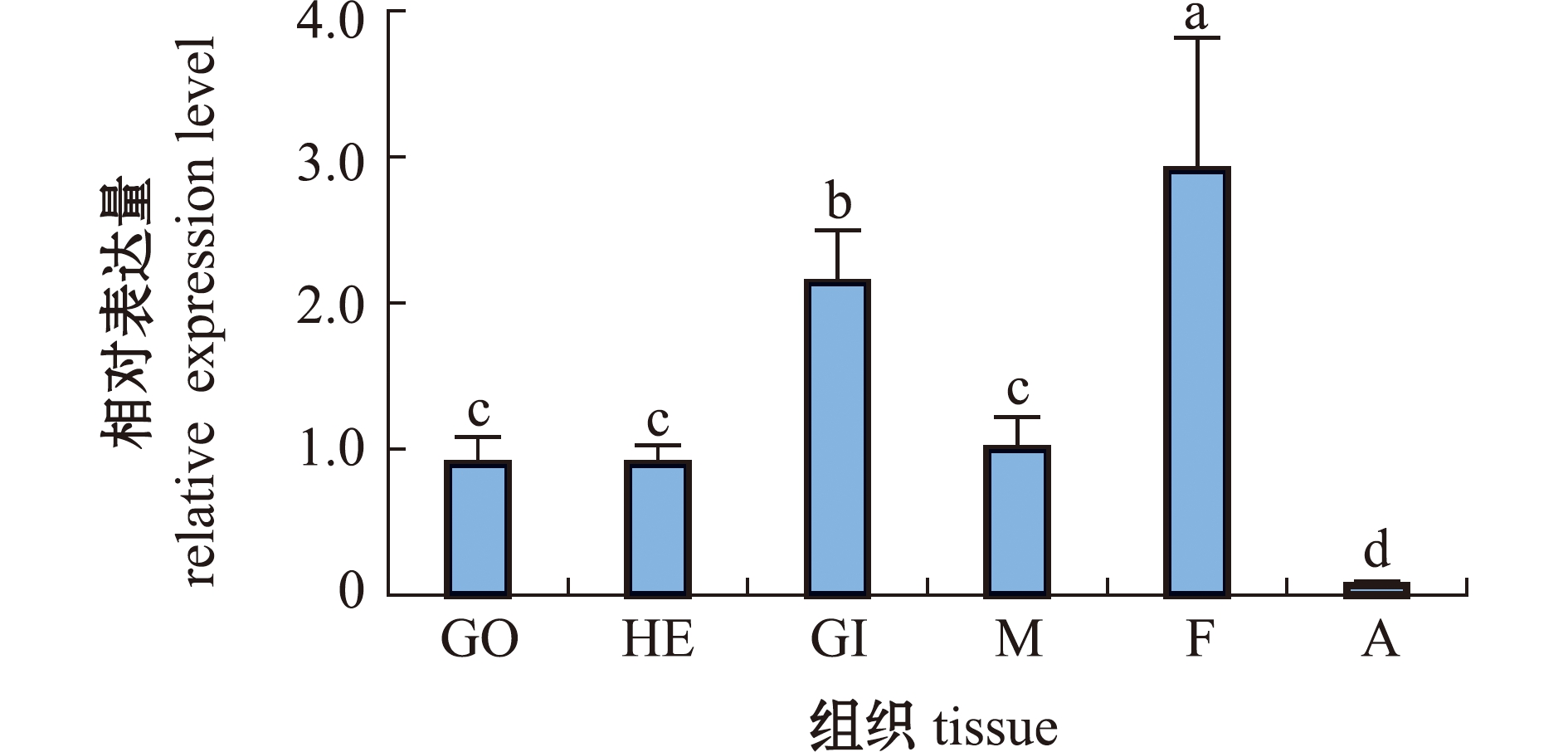
GO—性腺;HE—肝胰腺;GI—鳃;M—外套膜;F—足;A—闭壳肌。标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05)。
GO—gonad;HE—hepatopancreas;GI—gill;M—mantle;F—foot;A—adductor muscle. The means with different letters are significantly different in the groups at the 0.05 probability level,and the means with the same letter are not significant differences.
图3 α-Tubulin在马氏珠母贝不同组织中的相对表达量
Fig.3 Relative expression level of α-Tubulin in different tissues of Pinctada fucata martensii
2.4 马氏珠母贝鳃中α-Tubulin基因的时序表达
从图4可见,马氏珠母贝在低温胁迫下,α-Tubulin基因表达量呈先升高后降低的变化趋势,12、17 ℃低温组均在72 h时表达量达到最大值,且显著高于对照组(22 ℃)(P<0.05)。
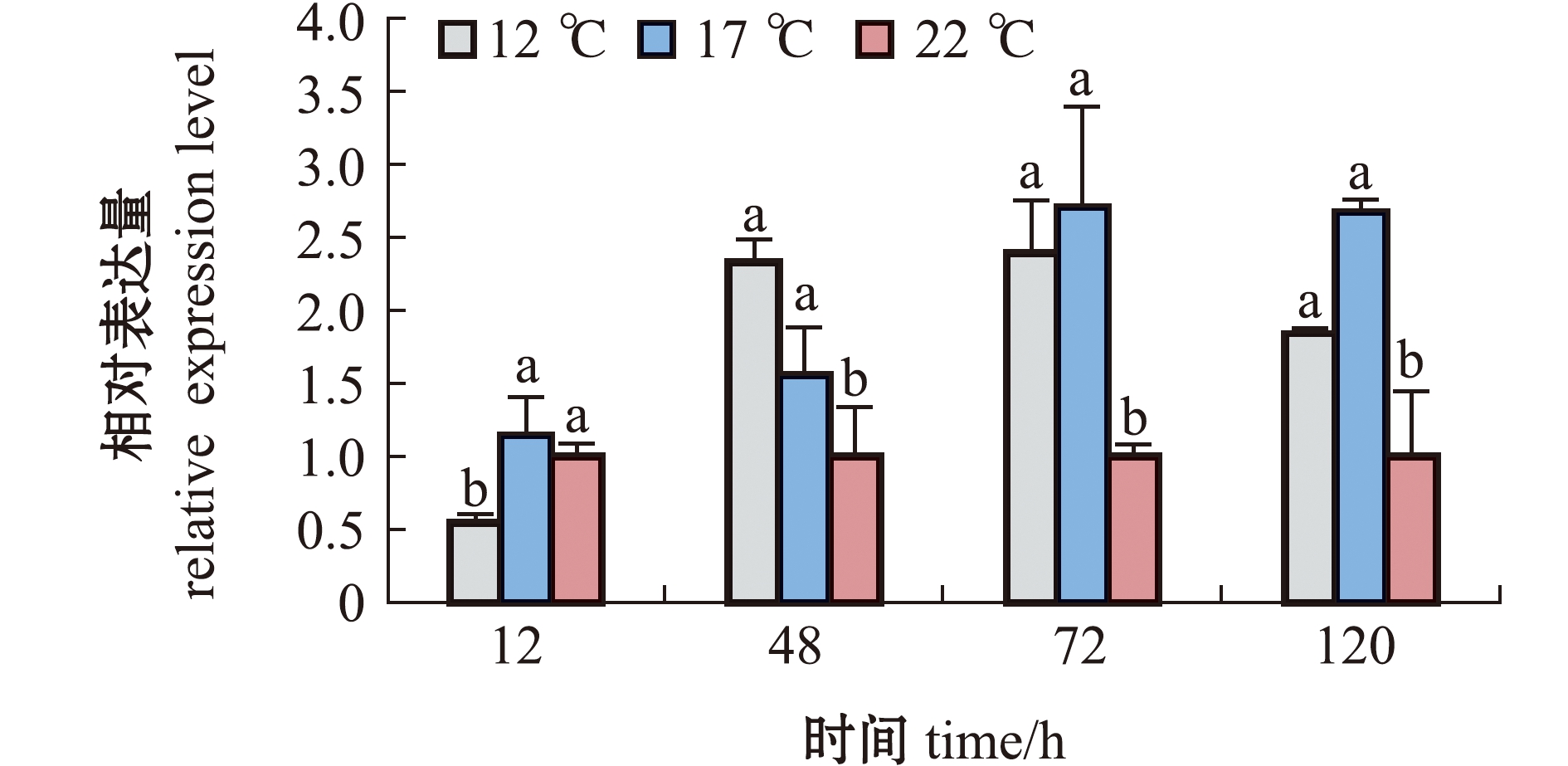
标有不同字母者表示同一时间点不同温度组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05)。
The means with different letters are significantly different in the groups at the 0.05 probability level,and the means with the same letter are not significant differences.
图4 12、17、22 ℃下马氏珠母贝鳃中α-Tubulin基因的时序表达
Fig.4 Temporal expression of the α-Tubulin gene in the gills of Pinctada fucata martensii at 12,17 ℃ and 22 ℃
2.5 α-Tubulin基因SNP位点的遗传多态性
α-Tubulin外显子具有34个SNP位点,对SNP位点的遗传信息分析显示:PIC为0.016 3~0.358 9,其中,低度多态性SNP位点(PIC<0.25)有26个,中度多态性SNP位点(0.25≤PIC<0.5)有8个;Ho为0.016 7~0.550 0;He为0.016 7~0.472 7(表2)。对α-Tubulin基因34个SNP位点的连锁不平衡分析发现,有5对位点完全连锁不平衡(D′=1,R2=1),91对强连锁不平衡(0.8≤D′<1,0.3≤R2<1),123对弱连锁不平衡(D′<0.8,R2<0.3)(图5)。

由白到红,颜色越深代表连锁程度越高。From white to red,the darker the color,the higher the linkage.
图5 α-Tubulin基因SNP位点的连锁不平衡分析
Fig.5 Linkage disequilibrium analysis for the SNP in α-Tubulin gene
表2 α-Tubulin基因的SNP多态性
Tab.2 Polymorphism analysis of SNP in α-Tubulin gene

序号No.位点position观测杂合度Ho期望杂合度He有效等位基因数Ne多态信息含量PIC哈迪-温伯格平衡HWE1g.1110686920.200 00.181 51.219 50.163 80.411 72g.1110687080.016 70.016 71.016 80.016 31.000 03g.1110687160.250 00.220 61.280 00.194 80.286 84g.1110687250.500 00.436 41.763 00.339 10.253 85g.1110687330.266 70.233 11.300 60.204 40.250 36g.1110687360.250 00.220 61.280 00.194 80.286 87g.1110687620.250 00.220 61.280 00.194 80.286 88g.1110687830.250 00.220 61.280 00.194 80.286 89g.1110693880.183 30.194 81.239 50.174 50.635 810g.1110694150.216 70.194 81.239 50.174 50.367 711g.1110694690.200 00.181 51.219 50.163 80.411 712g.1110694780.216 70.194 81.239 50.174 50.367 713g.1110698970.216 70.194 81.239 50.174 50.367 714g.1110699210.433 30.394 41.642 30.314 60.438 415g.1110699810.033 30.065 01.068 90.062 30.000 016g.1110702830.233 30.233 11.300 60.204 40.992 417g.1110703520.466 70.394 41.642 30.314 60.150 418g.1110705470.433 30.423 51.724 10.331 80.856 019g.1110705780.183 30.194 81.239 50.174 50.635 820g.1110706010.300 00.257 11.342 30.222 50.185 621g.1110706490.200 00.207 81.259 60.184 90.762 522g.1110706670.033 30.033 11.033 90.032 30.926 323g.1110710640.216 70.220 61.280 00.194 80.887 124g.1110711090.116 70.110 81.123 40.103 80.658 125g.1110711250.116 70.110 81.123 40.103 80.658 126g.1110711330.116 70.110 81.123 40.103 80.658 127g.1110711810.016 70.016 71.016 80.016 31.000 028g.1110711930.200 00.207 81.259 60.184 90.762 529g.1110735210.433 30.378 21.600 00.304 70.251 630g.1110735450.433 30.423 51.724 10.331 80.856 031g.1110736050.450 00.402 11.663 20.319 20.350 032g.1110736230.266 70.233 11.300 60.204 40.250 333g.1110743730.550 00.472 71.882 40.358 90.200 934g.1110743960.050 00.049 21.051 20.047 60.871 7
2.6 SNP位点与低温耐受性的关联分析
在34个SNP位点中,W和R群体中存在显著性差异的位点包括g.111071109、 g.111071125 和位点g.111071133,说明这3个位点可能与低温耐受性状相关,其中,位点g.111071109的基因型GT、位点g.111071125的基因型AC和位点g.111071133的基因型AG仅在R群体中被检测到(表3)。
表3 α-Tubulin基因SNP位点的基因型及等位基因在R和W群体间的分布频率
Tab.3 Distribution frequency of genotypes and alleles at SNPS of α-Tubulin genes between R and W populations
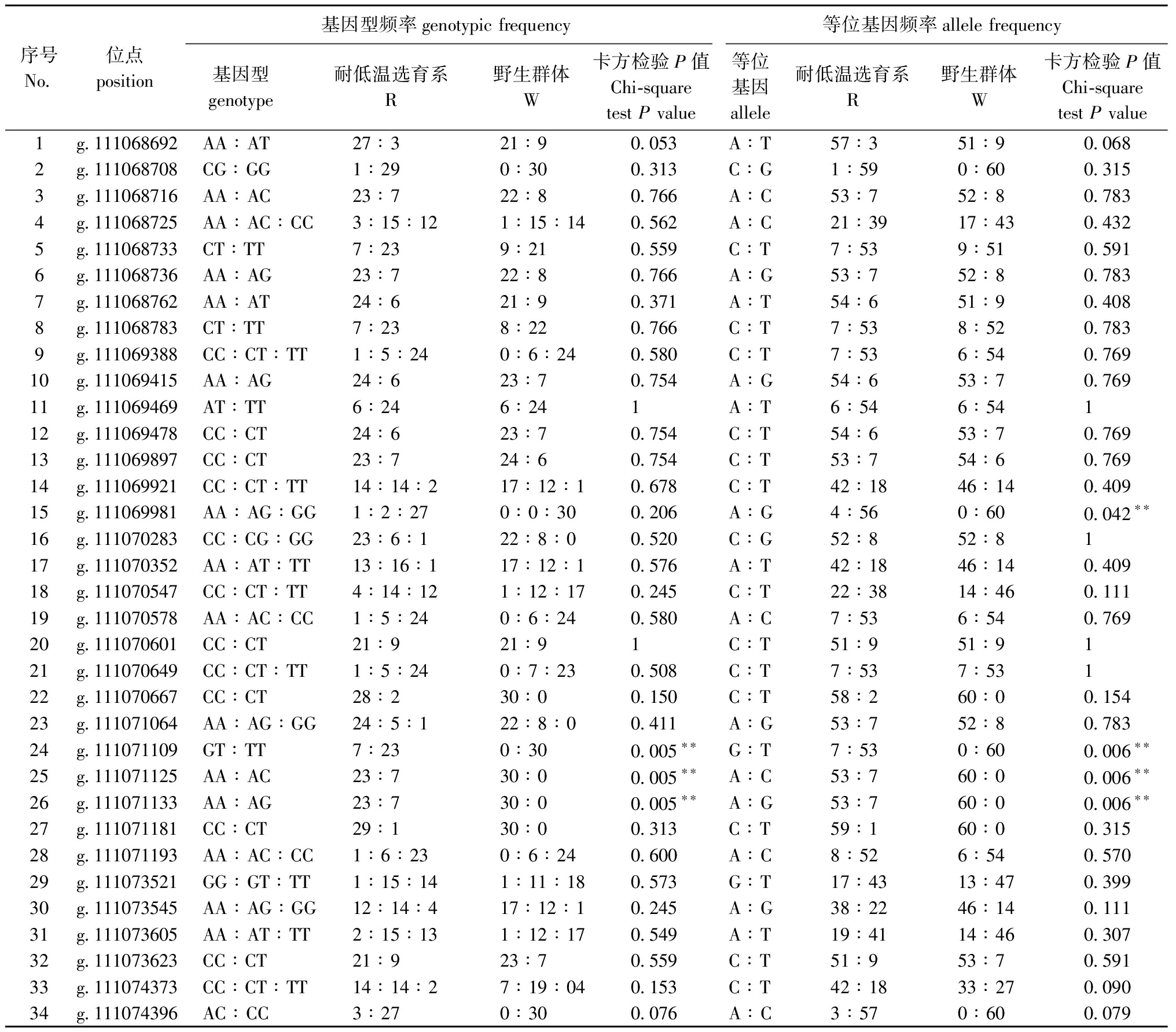
序号No.位点position基因型频率genotypic frequency等位基因频率allele frequency基因型genotype耐低温选育系R 野生群体W卡方检验P值Chi-square test P value等位基因allele耐低温选育系R 野生群体W卡方检验P值Chi-square test P value1g.111068692AA∶AT27∶321∶90.053A∶T57∶351∶90.0682g.111068708CG∶GG1∶290∶300.313C∶G1∶590∶600.3153g.111068716AA∶AC23∶722∶80.766A∶C53∶752∶80.7834g.111068725AA∶AC∶CC3∶15∶121∶15∶140.562A∶C21∶3917∶430.4325g.111068733CT∶TT7∶239∶210.559C∶T7∶539∶510.5916g.111068736AA∶AG23∶722∶80.766A∶G53∶752∶80.7837g.111068762AA∶AT24∶621∶90.371A∶T54∶651∶90.4088g.111068783CT∶TT7∶238∶220.766C∶T7∶538∶520.7839g.111069388CC∶CT∶TT1∶5∶240∶6∶240.580C∶T7∶536∶540.76910g.111069415AA∶AG24∶623∶70.754A∶G54∶653∶70.76911g.111069469AT∶TT6∶246∶241A∶T6∶546∶54112g.111069478CC∶CT24∶623∶70.754C∶T54∶653∶70.76913g.111069897CC∶CT23∶724∶60.754C∶T53∶754∶60.76914g.111069921CC∶CT∶TT14∶14∶217∶12∶10.678C∶T42∶1846∶140.40915g.111069981AA∶AG∶GG1∶2∶270∶0∶300.206A∶G4∶560∶600.042∗∗16g.111070283CC∶CG∶GG23∶6∶122∶8∶00.520C∶G52∶852∶8117g.111070352AA∶AT∶TT13∶16∶117∶12∶10.576A∶T42∶1846∶140.40918g.111070547CC∶CT∶TT4∶14∶121∶12∶170.245C∶T22∶3814∶460.11119g.111070578AA∶AC∶CC1∶5∶240∶6∶240.580A∶C7∶536∶540.76920g.111070601CC∶CT21∶921∶91C∶T51∶951∶9121g.111070649CC∶CT∶TT1∶5∶240∶7∶230.508C∶T7∶537∶53122g.111070667CC∶CT28∶230∶00.150C∶T58∶260∶00.15423g.111071064AA∶AG∶GG24∶5∶122∶8∶00.411A∶G53∶752∶80.78324g.111071109GT∶TT7∶230∶300.005∗∗G∶T7∶530∶600.006∗∗25g.111071125AA∶AC23∶730∶00.005∗∗A∶C53∶760∶00.006∗∗26g.111071133AA∶AG23∶730∶00.005∗∗A∶G53∶760∶00.006∗∗27g.111071181CC∶CT29∶130∶00.313C∶T59∶160∶00.31528g.111071193AA∶AC∶CC1∶6∶230∶6∶240.600A∶C8∶526∶540.57029g.111073521GG∶GT∶TT1∶15∶141∶11∶180.573G∶T17∶4313∶470.39930g.111073545AA∶AG∶GG12∶14∶417∶12∶10.245A∶G38∶2246∶140.11131g.111073605AA∶AT∶TT2∶15∶131∶12∶170.549A∶T19∶4114∶460.30732g.111073623CC∶CT21∶923∶70.559C∶T51∶953∶70.59133g.111074373CC∶CT∶TT14∶14∶27∶19∶040.153C∶T42∶1833∶270.09034g.111074396AC∶CC3∶270∶300.076A∶C3∶570∶600.079
注:P值为R和W群体分布频率的χ2检验(P<0.05);*表示P<0.05;**表示P<0.01,下同。
Note:P values,χ2 test for R and W populations distribution frequencies (P<0.05);*,P<0.05;**,P<0.01,et sequentia.
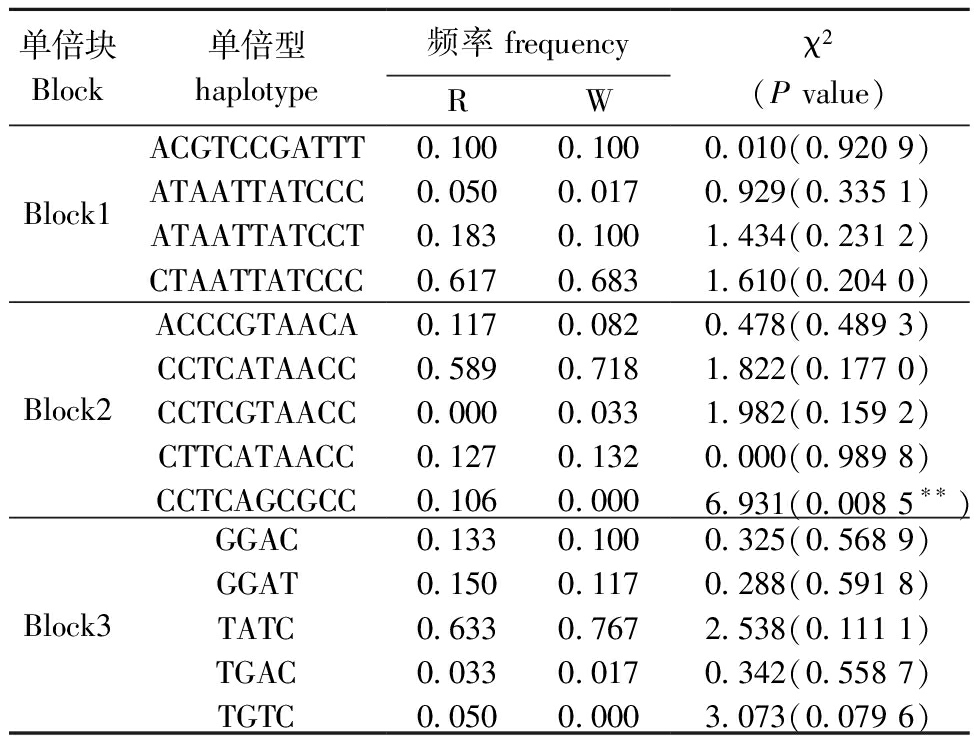
利用Haploview 4.2软件对α-Tubulin外显子区的SNP进行连锁不平衡分析,结果显示,34个位点构成3个单倍块和14种单倍型,其中,单倍型CCTCAGCGCC与马氏珠母贝耐低温性状显著相关(P<0.05)(表4)。
表4 α-Tubulin基因单倍型在R和W群体中的出现频率
Tab.4 Frequency of α-Tubulin haplotypes in R and W populations
单倍块 Block单倍型 haplotype频率 frequencyRWχ2(P value)Block1ACGTCCGATTT0.1000.1000.010(0.920 9)ATAATTATCCC0.0500.0170.929(0.335 1)ATAATTATCCT0.1830.1001.434(0.231 2)CTAATTATCCC0.6170.6831.610(0.204 0)Block2ACCCGTAACA0.1170.0820.478(0.489 3)CCTCATAACC0.5890.7181.822(0.177 0)CCTCGTAACC0.0000.0331.982(0.159 2)CTTCATAACC0.1270.1320.000(0.989 8)CCTCAGCGCC0.1060.0006.931(0.008 5∗∗)Block3GGAC0.1330.1000.325(0.568 9)GGAT0.1500.1170.288(0.591 8)TATC0.6330.7672.538(0.111 1)TGAC0.0330.0170.342(0.558 7)TGTC0.0500.0003.073(0.079 6)
3 讨论
3.1 马氏珠母贝α-Tubulin基因编码的蛋白结构
本试验中,通过RACE技术扩增得到了马氏珠母贝α-Tubulin的全长序列,序列分析表明,马氏珠母贝α-Tubulin氨基酸序列具有α-Tubulin保守区GGGTGSG,该位点为GTP核苷酸结合位点,是聚合α、β-微管蛋白的关键位点[18];编码的蛋白具有Tubulin、Tubulin-C两个完整的结构域。在马氏珠母贝α-Tubulin N端也发现了对微管蛋白转录后调控非常重要的保守序列MRECI[19-20];C末端保守的酪氨酸也存在于马氏珠母贝α-Tubulin中,该酪氨酸涉及α-Tubulin的酪氨酸化及去酪氨酸化[21]。此外,进化树也表明,α-Tubulin在整个物种分化过程中较为保守。
3.2 马氏珠母贝鳃中α-Tubulin基因的表达与低温胁迫的相关性分析
微管结构可调节细胞的物质运输,影响细胞膜通道的离子活性,在逆境应答中也起着重要作用[2-3]。温度对微管结构影响极大,在0~4 ℃时会产生解聚现象,利于微管组装的温度为25 ℃以上[22]。低温胁迫可引起微管解聚并常伴随植株的伤害,且微管的冷稳定性与植物的耐寒程度有一定的正相关[23-24]。本研究表明,温度胁迫及胁迫时间均与马氏珠母贝α-Tubulin的表达存在较强的相关性。通过对鳃组织中α-Tubulin在不同温度下的时序表达分析发现,12、17 ℃低温组该基因的表达量均在72 h时达到最高,且显著高于对照组。由此推测,α-Tubulin在低温下高表达与马氏珠母贝修复低温损伤组织相关。
α-Tubulin作为真核生物管家基因的重要成员之一,因其表达具有稳定性,常被选为内参基因[25-26]。研究发现,虾夷扇贝(Patinopecten yessoensis)α-Tubulin在饥饿状态、急性感染研究中能作为内参基因,但在升温条件下则不可[27]。本研究表明,α-Tubulin在马氏珠母贝足和鳃组织中显著高表达,且在低温胁迫后表达上调。这提示,在珍珠贝相关研究中,α-Tubulin不应作为内参基因使用。
3.3 马氏珠母贝鳃中α-Tubulin基因SNP/单倍型与耐低温的关联分析
基因中的SNP与快速生长、抗病性和免疫反应等生物性状密切相关[28-30]。本研究中,在马氏珠母贝α-Tubulin的外显子区发现了34个SNP。哈温平衡分析显示,g.111069981不符合哈温平衡(P<0.05),SNP偏离哈温平衡现象的出现,可以作为与抗性相关的初步推断[31]。R群体和W群体的基因型频率分析显示,g.111071109、g.111071125和g.111071133这3个SNP位点的基因型频率在两个群体间存在显著性差异,表明这些SNP位点可能与马氏珠母贝对低温的抗性有关。3个位点中g.111071109和g.111071133属于同义突变,g.111071125属于非同义突变。非编码区的SNP位点主要在转录阶段影响转录因子的结合、剪接位点,而编码区的SNP可以改变蛋白质的氨基酸从而影响其功能[32]。在g.111071125位点,天冬酰胺(Asn)变为组氨酸(His),天冬酰胺是极性的中性氨基酸,而组氨酸是碱性氨基酸,因此,该位点的突变可能由此对蛋白功能造成影响,进而提高马氏珠母贝对低温的耐受能力。
单倍型水平分析被认为比单标记等位基因水平分析更稳健[33]。对α-Tubulin外显子区的SNP进行连锁不平衡分析显示,34个位点构成3个单倍块和14种单倍型,其中,单倍型CCTCAGCGCC的频率在R群体明显高于W群体。这些数据表明,α-Tubulin基因在马氏珠母贝低温适应过程中发挥了作用,与抗性相关的SNP/单倍型可能有助于选育马氏珠母贝耐低温品系。
4 结论
1)马氏珠母贝α-Tubulin基因在低温胁迫后表达量显著上调,表明该基因与马氏珠母贝修复低温损伤组织相关。
2)马氏珠母贝R群体和W群体α-Tubulin基因SNP/单倍型分析显示,SNP位点g.111071125及优异单倍型CCTCAGCGCC可作为以耐低温为育种目标的候选标记。
[1] BUENO O,TOBAJAS G,QUESADA E,et al.Conformational mimetics of the α-methyl chalcone TUB091 binding Tubulin:design,synthesis and antiproliferative activity[J].European Journal of Medicinal Chemistry,2018,148:337-348.
[2] 杨洪,邓治,刘辉,等.巴西橡胶树α-微管蛋白HbTUA2基因的克隆、表达及生物信息学分析[J].植物生理学报,2017,53(1):52-62.
YANG H,DENG Z,LIU H,et al.Cloning,expression and bioinformatics analysis of HbTUA2 gene in Hevea brasiliensis(rubber tree)[J].Plant Physiology Journal,2017,53(1):52-62.(in Chinese)
[3] 李琦瑶,周培禄,王树声,等.烟草β-微管蛋白基因NtTubB的克隆及逆境响应分析[J].分子植物育种,2019,17(13):4210-4219.
LI Q Y,ZHOU P L,WANG S S,et al.Cloning and abiotic stress response analysis of β-Tubulin gene NtTubB in Nicotiana tabacum[J].Molecular Plant Breeding,2019,17(13):4210-4219.(in Chinese)
[4] DESHAYES F,FRADET M,KAMINSKI S,et al.Link between the EZH2 noncanonical pathway and microtubule organization center polarization during early T lymphopoiesis[J].Scientific Reports,2022,12(1):3655.
[5] WANG H M,WANG Y,NIU M H,et al.Cold acclimation for enhancing the cold tolerance of zebrafish cells[J].Frontiers in Physiology,2021,12:813451.
[6] TSIOLI S,KOUTALIANOU M,GKAFAS G A,et al.Responses of the Mediterranean seagrass Cymodocea nodosa to combined temperature and salinity stress at the ionomic,transcriptomic,ultrastructural and photosynthetic levels[J].Marine Environmental Research,2022,175:105512.
[7] 王怡,高银雪,湛垚垚,等.α-Tubulin基因的克隆、生物信息学分析及其在仿刺参肠道再生过程中的表达模式[J].水产学报,2016,40(4):547-557.
WANG Y,GAO Y X,ZHAN Y Y,et al.Molecular cloning,bioinformatics analysis and expression pattern detection of α-Tubulin gene during intestinal regeneration in the sea cucumber(Apostichopus japonicus)[J].Journal of Fisheries of China,2016,40(4):547-557.(in Chinese)
[8] ALI D N,ABBADY A Q,KWEIDER M,et al.Cloning,expression,purification and characterization of Leishmania tropica PDI-2 protein[J].Open Life Sciences,2016,11(1):166-176.
[9] CORVAISIER M,ZHOU J K,MALYCHEVA D,et al.The γ-Tubulin meshwork assists in the recruitment of PCNA to chromatin in mammalian cells[J].Communications Biology,2021,4(1):767.
[10] 王软林,肖羽,李佳,等.八肋游仆虫微管蛋白基因家族的鉴定及进化分析[J].水生生物学报,2021,45(5):1005-1013.
WANG R L,XIAO Y,LI J,et al.Identification and evolution analysis of Tubulin superfamily genes in euplotes octocarinatus[J].Acta Hydrobiologica Sinica,2021,45(5):1005-1013.(in Chinese)
[11] BIENIUSSA L,JAIN I,BOSCH GRAU M,et al.Microtubule and auditory function-an underestimated connection[J].Seminars in Cell &Developmental Biology,2023,137:74-86.
[12] TAMAYO N A,BOURBEAU M P,ALLEN J R,et al.Targeting the mitotic kinesin KIF18A in chromosomally unstable cancers:hit optimization toward an in vivo chemical probe[J].Journal of Medicinal Chemistry,2022,65(6):4972-4990.
[13] WANG Q H,LIU Y,ZHENG Z,et al.Adaptive response of pearl oyster Pinctada fucata martensii to low water temperature stress[J].Fish &Shellfish Immunology,2018,78:310-315.
[14] 余云登,朱浩拥,王盛南,等.暗纹东方鲀leptin基因的克隆、SNP筛选及其与生长性状的关联分析[J].大连海洋大学学报,2021,36(2):207-213.
YU Y D,ZHU H Y,WANG S N,et al.Cloning,SNP screening and association with growth traits of leptin gene in river puffer Takifugu obscurus[J].Journal of Dalian Ocean University,2021,36(2):207-213.(in Chinese)
[15] WANG P L,DAI M H,XUAN W J,et al.SNP function portal:a web database for exploring the function implication of SNP alleles[J].Bioinformatics,2006,22(14):e523-e529.
[16] DENG Y W,LEI Q N,TIAN Q L,et al.De novo assembly, gene annotation, and simple sequence repeat marker development using Illumina paired-end transcriptome sequences in the pearl oyster Pinctada maxima[J].Bioscience Biochemistry and Biotechnology,2014,78(10): 1685-1692.
[17] 赖卓欣.马氏珠母贝耐低温选育系的选择印记分析[D].湛江:广东海洋大学,2020.
LAI Z X.Genome selection sweep of low temperature resistant line from Pinctada fucata martensii[D].Zhanjiang:Guangdong Ocean University,2020.(in Chinese)
[18] KIRSCHNER M,MITCHISON T.Beyond self-assembly:from microtubules to morphogenesis[J].Cell,1986,45(3):329-342.
[19] YEN T J,GAY D A,PACHTER J S,et al.Autoregulated changes in stability of polyribosome-bound beta-Tubulin mRNAs are specified by the first 13 translated nucleotides[J].Molecular and Cellular Biology,1988,8(3):1224-1235.
[20] YEN T J,MACHLIN P S,CLEVELAND D W.Autoregulated instability of β-Tubulin mRNAs by recognition of the nascent amino terminus of β-Tubulin[J].Nature,1988,334(6183):580-585.
[21] BULINSKI J C,GUNDERSEN G G.Stabilization of post-translational modification of microtubules during cellular morphogenesis[J].Bioessays,1991,13(6):285-293.
[22] RIKIN A,ATSMON D,GITLER C.Chilling injury in cotton (Gossypium hirsutum L.):effects of antimicrotubular drugs[J].Plant and Cell Physiology,1980,21(5):829-837.
[23] 李永才,安黎哲,毕阳.微管骨架在植物适应低温胁迫中的功能研究进展[J].西北植物学报,2006,26(7):1500-1504.
LI Y C,AN L Z,BI Y.Research advances about the role of microtubule cytoskeleton in plant acclimation to low-temperature stress[J].Acta Botanica Boreali-Occidentalia Sinica,2006,26(7):1500-1504.(in Chinese)
[24] RIKIN A,ATSMON D,GITLER C.Quantitation of chill-induced release of a tubulin-like factor and its prevention by abscisic acid in Gossypium hirsutum L.[J].Plant Physiology,1983,71(4):747-748.
[25] ZHENG W J,SUN L.Evaluation of housekeeping genes as references for quantitative real time RT-PCR analysis of gene expression in Japanese flounder (Paralichthys olivaceus)[J].Fish &Shellfish Immunology,2011,30(2):638-645.
[26] ZHAO Y,CHEN M Y,WANG T M.Selection of reference genes for qRT-PCR analysis of gene expression in sea cucumber Apostichopus japonicus during aestivation[J].Chinese Journal of Oceanology and Limnology,2014,32(6):1248-1256.
[27] 鲍相渤,刘卫东,姜冰,等.内参基因在虾夷扇贝定量PCR中表达稳定性的比较[J].水产科学,2011,30(10):603-608.
BAO X B,LIU W D,JIANG B,et al.Expression stability of reference genes for quantitative PCR in Japanese scallop Patinopecten yessoensis[J].Fisheries Science,2011,30(10):603-608.(in Chinese)
[28] 杨创业,张丞澍,黄静,等.马氏珠母贝Kelch-like基因的序列及其SNP位点分析[J].大连海洋大学学报,2022,37(3):386-393.
YANG C Y,ZHANG C S,HUANG J,et al.Sequence and SNP analysis of Kelch-like gene from pearl oyster Pinctada fucata martensii[J].Journal of Dalian Ocean University,2022,37(3):386-393.(in Chinese)
[29] XU Z L,TAYLOR J A.SNPinfo:integrating GWAS and candidate gene information into functional SNP selection for genetic association studies[J].Nucleic Acids Research,2009,37(sup l/2):W600-W605.
[30] WANG Z,MOULT J.SNPs,protein structure,and disease[J].Human Mutation,2001,17(4):263-270.
[31] 曹建萌,胡欣欣,卢迈新,等.尼罗罗非鱼补体C9基因单核苷酸多态性及其与抗无乳链球菌感染的关联分析[J].农业生物技术学报,2017,25(3):354-365.
CAO J M,HU X X,LU M X,et al.SNPs of C9 gene and their association with resistance to the infection of Streptococcus agalactiae in Oreochromis niloticus[J].Journal of Agricultural Biotechnology,2017,25(3):354-365.(in Chinese)
[32] REUMERS J,CONDE L,MEDINA I,et al.Joint annotation of coding and non-coding single nucleotide polymorphisms and mutations in the SNP effect and PupaSuite databases[J].Nucleic Acids Research,2008,36(1):D825-D829.
[33] CLARK A G.The role of haplotypes in candidate gene studies[J].Genetic Epidemiology,2004,27(4):321-333.