中国拥有世界上最大的贝类养殖产业,据《2022中国渔业统计年鉴》,2021年全国海水养殖贝类产量为1 526.07万t,占海水养殖产量的69.01%。随着中国贝类养殖产业的不断发展,病害问题日显突出,2020年,中国养殖贝类因病害造成的经济损失达120亿元。炎症反应是机体重要的免疫防御机制,能及时清除入侵的病原菌,然而,过度的炎症反应会造成机体组织损伤甚至引发个体死亡。本文围绕贝类炎症细胞因子及其生物学功能、炎症相关信号通路及炎症反应的形态学特征,总结了近年来贝类炎症的研究进展,并重点阐述了炎症发生的分子机制,对进一步深入认识贝类的免疫防御机制,了解炎症反应的进化过程具有一定的理论意义,同时对贝类病害防控及推动贝类养殖产业绿色高质量发展具有参考价值。
1 贝类炎症细胞因子及其生物学功能
炎症细胞因子是由细胞分泌的在细胞间发挥调节作用的一类小分子可溶性多肽蛋白。根据其结构和生物学功能,炎症细胞因子可以分为白介素(interleukin,IL)、肿瘤坏死因子(tumour necrosis factor,TNF)、干扰素(interferon,IFN)和趋化因子(chemokine)等[1-2]。目前,在贝类中已经鉴定出IL、TNF、IFN样蛋白(IFN-like protein,IFNLP)、巨噬细胞迁移抑制因子(macrophage migration inhibitory factor,MIF)、高迁移率族蛋白1(high mobility group box 1,HMGB1)和同种移植炎症因子-1(allograft inflammatory factor-1,AIF1)等炎症细胞因子[3]。
1.1 IL及其生物学功能
IL是一类由免疫细胞分泌的在细胞间起免疫调节作用的小分子蛋白,根据发现的先后顺序被命名为IL1、IL2、IL3等。目前,在贝类中仅发现了两类IL,即IL12[4]和IL17[5-8](表1)。其中,IL12仅在长牡蛎(Crassostrea gigas)中被发现。CgIL12p35L属于IL12的p35样亚型,含有4个螺旋链,其mRNA主要在闭壳肌和外套膜中表达。在鳗弧菌(Vibrio anguillarum)刺激后,CgIL12p35L在血淋巴细胞中的mRNA表达水平显著升高,其重组蛋白可以促进血淋巴对鳗弧菌和大肠杆菌(Escherichia coli)的清除[4]。在长牡蛎、紫贻贝(Mytilus galloprovincialis)、马氏珠母贝(Pinctada fucata)和三角帆蚌(Hyriopsis cumingii)中已经鉴定出IL17(表1)[5-6,9-10]。在长牡蛎中共发现10个IL17(CgIL17-1/2/3/4/5/6/7/8/9/10)。其中,CgIL17-1/2/3/4/5/6的mRNA在多个组织中均有表达,在鳃中的表达水平相对较高[6]。在紫贻贝中共发现6个IL17(MgIL17-1/2/3/4/5/6)。其中,MgIL17-1/2/3/4/5含有一个信号肽和一个IL17结构域,而MgIL17-6只含有一个IL17结构域。它们的mRNA在消化腺、鳃和外套膜等多个组织中均有表达[10]。在马氏珠母贝和三角帆蚌中分别鉴定出一个IL17,即PfIL17和HcIL17,其中,HcIL17在消化腺和鳃中的mRNA表达水平相对较高[5,9]。贝类中的IL17在调节炎症反应和固有免疫应答中发挥重要作用。如在长牡蛎中,CgIL17-1可以与其受体CgIL17R1结合,促进血淋巴细胞的增殖[11]。CgIL17-5重组蛋白具有直接结合脂多糖(lipopolysaccharide,LPS)、肽聚糖(peptidoglycan,PGN)、聚肌胞苷酸[Poly(I:C)]和葡聚糖(glucan,Glu)的功能,并能显著抑制藤黄微球菌(Micrococcus luteus)和大肠杆菌(Esherichia coli)的生长[12];CgIL17-5重组蛋白还可以促进丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)磷酸化[13]和转录因子NF-κB/Rel、AP-1转位入核,诱导炎症细胞因子的mRNA表达,并引发鳃组织肿胀、纤毛脱落和血淋巴细胞浸润现象。三角帆蚌的HcIL17在消化腺和鳃中的mRNA表达水平相对较高,其mRNA表达水平在同种异体移植试验中被显著诱导[9]。因此,贝类的IL17可以作为前炎症细胞因子,诱导多种细胞因子的表达。但贝类中不同的IL17激活的下游信号通路和调控的免疫效应不同,在贝类抗感染免疫中发挥的作用也不同。
表1 贝类炎症细胞因子及其在不同组织中的分布情况
Tab.1 Inflammatory factors and their tissue distribution in molluscs
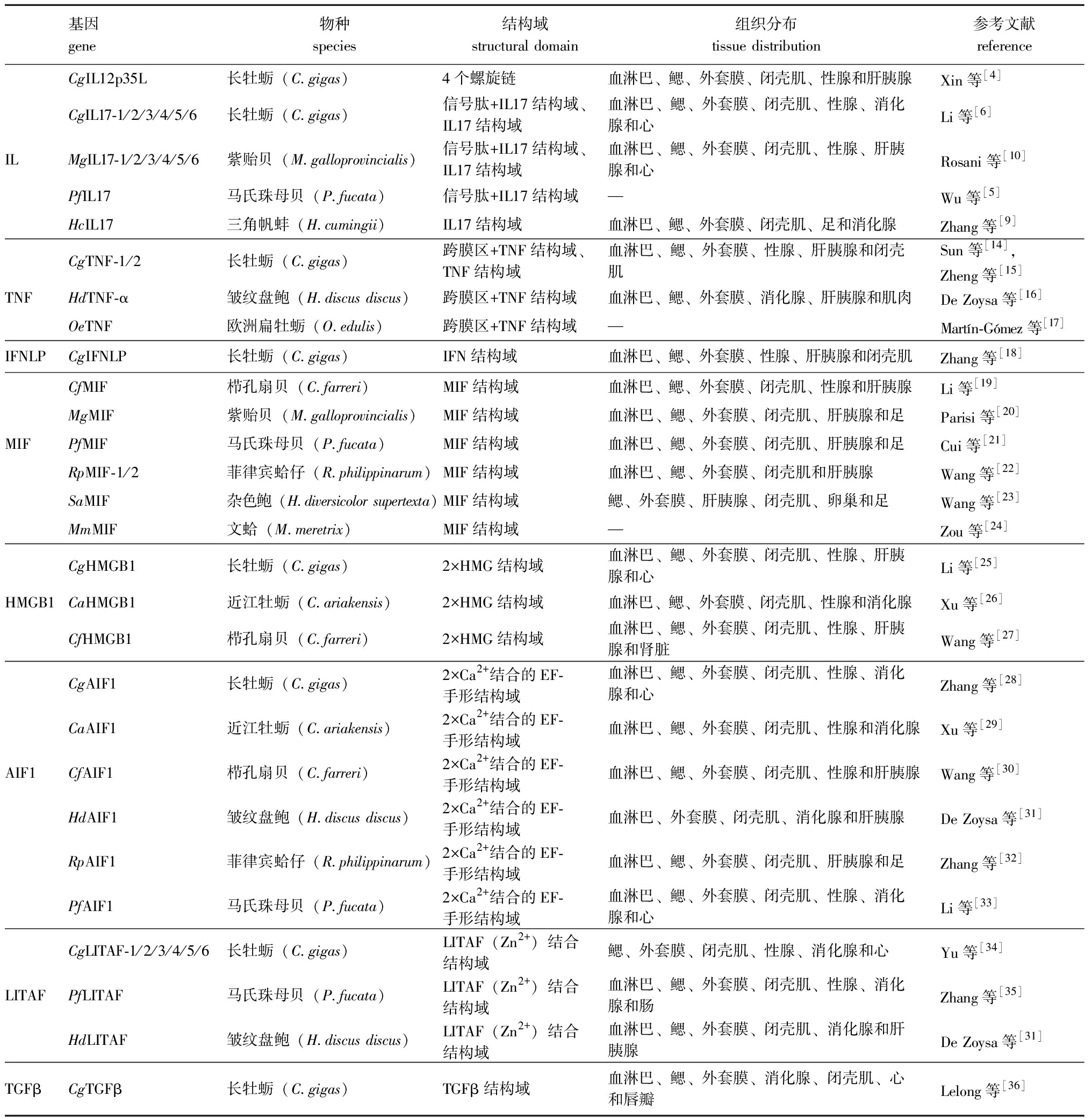
基因 gene物种 species结构域structural domain组织分布 tissue distribution参考文献referenceCgIL12p35L长牡蛎(C.gigas)4个螺旋链血淋巴、鳃、外套膜、闭壳肌、性腺和肝胰腺Xin等[4]CgIL17-1/2/3/4/5/6长牡蛎(C.gigas)信号肽+IL17结构域、IL17结构域血淋巴、鳃、外套膜、闭壳肌、性腺、消化腺和心Li等[6]ILMgIL17-1/2/3/4/5/6紫贻贝(M.galloprovincialis)信号肽+IL17结构域、IL17结构域血淋巴、鳃、外套膜、闭壳肌、性腺、肝胰腺和心Rosani等[10]PfIL17马氏珠母贝(P.fucata)信号肽+IL17结构域—Wu等[5]HcIL17三角帆蚌(H.cumingii)IL17结构域血淋巴、鳃、外套膜、闭壳肌、足和消化腺Zhang等[9]CgTNF-1/2长牡蛎(C.gigas)跨膜区+TNF结构域、TNF结构域血淋巴、鳃、外套膜、性腺、肝胰腺和闭壳肌Sun等[14],Zheng等[15]TNFHdTNF-α皱纹盘鲍(H.discus discus)跨膜区+TNF结构域血淋巴、鳃、外套膜、消化腺、肝胰腺和肌肉De Zoysa等[16]OeTNF欧洲扁牡蛎(O.edulis)跨膜区+TNF结构域—Martín-Gómez等[17]IFNLPCgIFNLP长牡蛎(C.gigas)IFN结构域血淋巴、鳃、外套膜、性腺、肝胰腺和闭壳肌Zhang等[18]CfMIF栉孔扇贝(C.farreri)MIF结构域血淋巴、鳃、外套膜、闭壳肌、性腺和肝胰腺Li等[19]MgMIF紫贻贝(M.galloprovincialis)MIF结构域血淋巴、鳃、外套膜、闭壳肌、肝胰腺和足Parisi等[20]MIFPfMIF马氏珠母贝(P.fucata)MIF结构域血淋巴、鳃、外套膜、闭壳肌、肝胰腺和足Cui等[21]RpMIF-1/2菲律宾蛤仔(R.philippinarum)MIF结构域血淋巴、鳃、外套膜、闭壳肌和肝胰腺Wang等[22]SaMIF杂色鲍(H.diversicolor supertexta)MIF结构域鳃、外套膜、肝胰腺、闭壳肌、卵巢和足Wang等[23]MmMIF文蛤(M.meretrix)MIF结构域—Zou等[24]CgHMGB1长牡蛎(C.gigas)2×HMG结构域血淋巴、鳃、外套膜、闭壳肌、性腺、肝胰腺和心Li等[25]HMGB1CaHMGB1近江牡蛎(C.ariakensis)2×HMG结构域血淋巴、鳃、外套膜、闭壳肌、性腺和消化腺Xu等[26]CfHMGB1栉孔扇贝(C.farreri)2×HMG结构域血淋巴、鳃、外套膜、闭壳肌、性腺、肝胰腺和肾脏Wang等[27]CgAIF1长牡蛎(C.gigas)2×Ca2+结合的EF-手形结构域血淋巴、鳃、外套膜、闭壳肌、性腺、消化腺和心Zhang等[28]CaAIF1近江牡蛎(C.ariakensis)2×Ca2+结合的EF-手形结构域血淋巴、鳃、外套膜、闭壳肌、性腺和消化腺Xu等[29]AIF1CfAIF1栉孔扇贝(C.farreri)2×Ca2+结合的EF-手形结构域血淋巴、鳃、外套膜、闭壳肌、性腺和肝胰腺Wang等[30]HdAIF1皱纹盘鲍(H.discus discus)2×Ca2+结合的EF-手形结构域血淋巴、外套膜、闭壳肌、消化腺和肝胰腺De Zoysa等[31]RpAIF1菲律宾蛤仔(R.philippinarum)2×Ca2+结合的EF-手形结构域血淋巴、鳃、外套膜、闭壳肌、肝胰腺和足Zhang等[32]PfAIF1马氏珠母贝(P.fucata)2×Ca2+结合的EF-手形结构域血淋巴、鳃、外套膜、闭壳肌、性腺、消化腺和心Li等[33]CgLITAF-1/2/3/4/5/6长牡蛎(C.gigas)LITAF(Zn2+)结合结构域鳃、外套膜、闭壳肌、性腺、消化腺和心Yu等[34]LITAFPfLITAF马氏珠母贝(P.fucata)LITAF(Zn2+)结合结构域血淋巴、鳃、外套膜、闭壳肌、性腺、消化腺和肠Zhang等[35]HdLITAF皱纹盘鲍(H.discus discus)LITAF(Zn2+)结合结构域血淋巴、鳃、外套膜、闭壳肌、消化腺和肝胰腺De Zoysa等[31]TGFβCgTGFβ长牡蛎(C.gigas)TGFβ结构域血淋巴、鳃、外套膜、消化腺、闭壳肌、心和唇瓣Lelong等[36]
1.2 TNF及其生物学功能
TNF是一种具有多种生物学效应的细胞因子。按其来源不同,TNF分为由单核/巨噬细胞分泌的TNF-α和由活化的T淋巴细胞分泌的TNF-β。目前,在贝类中已鉴定出TNF同源分子,它们在多个组织中均有表达[14-17,37](表1)。在长牡蛎基因组中共发现23个TNF[38],其中,CgTNF-1和CgTNF-2被进一步克隆鉴定。CgTNF-2在血淋巴中的mRNA表达水平相对较高[15]。在皱纹盘鲍(Haliotis discus discus)和欧洲扁牡蛎(Ostrea edulis)中分别鉴定出一个TNF,即HdTNF-α和OeTNF。HdTNF-α的mRNA主要在血淋巴、鳃和外套膜中表达[16],OeTNF主要在鳃中表达[17]。贝类的TNF在促进炎症反应和调节固有免疫应答中发挥重要作用。如长牡蛎CgTNF-1的重组蛋白可促进血淋巴细胞的增殖、凋亡和吞噬,同时其还具有一定的抗菌活性[14,37];CgTNF-2在血淋巴中的mRNA表达水平相对较高,其重组蛋白可抑制A549细胞的增殖和血淋巴中灿烂弧菌(Vibrio splendidus)的生长[15]。这些研究表明,贝类中TNF在调节细胞命运和抗感染免疫中发挥重要作用。
1.3 IFNLP及其生物学功能
干扰素是一类多功能细胞因子,在固有免疫应答中发挥重要作用。依据产生干扰素的细胞类型、理化性质和生物学活性等方面的差异,干扰素可分为Ⅰ、Ⅱ和Ⅲ型。在无脊椎动物中,目前仅在长牡蛎中发现了一个含有干扰素结构域的类IFN分子CgIFNLP[18](表1),该分子的氨基酸序列与高等动物的IFN相似度较低。CgIFNLP的mRNA主要在鳃中表达,其重组蛋白可以促进血淋巴细胞发生凋亡、吞噬,并抑制A549细胞增殖[18]。CgIFNLP同样可以激活机体的抗病毒免疫反应。如CgIFNLP可以促进血淋巴细胞中抗病毒分子CgIFI44L-1和CgMx1的表达[39-40]。这些研究表明,在贝类中已经存在具有抗病毒功能的类干扰素分子。
1.4 MIF及其生物学功能
MIF最初被认为是一种T淋巴细胞来源的淋巴因子。但越来越多的研究表明,MIF不仅具有抑制巨噬细胞迁移的作用,还具有促炎活性。目前,MIF在栉孔扇贝(Chlamys farreri)[19]、紫贻贝[20]、马氏珠母贝[21]、菲律宾蛤仔(Ruditapes philippinarum)[22]、杂色鲍(H.diversicolor supertexta)[23] 和文蛤(Meretrix meretrix)[24]中被发现。它们含有一个MIF结构域,且在多个组织中均有表达(表1)。其中,栉孔扇贝CfMIF在肝胰腺中的mRNA表达水平相对较高,其次是外套膜和鳃[19];紫贻贝MgMIF的mRNA主要在血淋巴和外套膜中表达[20];马氏珠母贝PfMIF在肝胰腺、性腺和肠中的mRNA表达水平相对较高[21]。贝类的MIF可以响应免疫刺激,在调节免疫应答和炎症反应中发挥作用。如栉孔扇贝的CfMIF重组蛋白可以促进羊成纤维细胞的迁移[19];在溶藻弧菌(V.alginolyticus)刺激后,马氏珠母贝PfMIF在消化腺、血淋巴细胞和肠中的mRNA表达水平显著升高[21];在鳗弧菌或藤黄微球菌刺激后,菲律宾蛤仔的RpMIF重组蛋白具有互变异构酶和氧化还原酶活性,提示其参与机体的炎症反应[22]。这些研究表明,贝类的MIF主要在免疫相关组织中高表达,且可以响应不同病原菌刺激,提示它们在调控贝类抗感染免疫中发挥重要作用。
1.5 HMGB1及其生物学功能
HMGB1是一种高度保守的核蛋白,在调节机体免疫应答中发挥重要作用。目前,仅在长牡蛎[25,41]、近江牡蛎(C.ariakensis)[26]和栉孔扇贝[27]中发现了该分子,它们含有两个HMG结构域,且在多个组织中有表达(表1)。其中,长牡蛎CgHMGB1和近江牡蛎CaHMGB1在血淋巴中的mRNA表达水平相对较高[25-26],栉孔扇贝CfHMGB1的mRNA主要在肝胰腺和外套膜中表达[27]。贝类的HMGB1在抗感染免疫中发挥重要作用。如长牡蛎的CgHMGB1重组蛋白具有结合细菌和多糖的功能,该蛋白也可以分泌到血清中,并抑制灿烂弧菌和大肠杆菌的生长。CgHMGB1重组蛋白还可以促进血淋巴细胞中CgMAPK磷酸化和CgRel转位入核,并诱导多种炎症细胞因子的表达和鳃组织发生肿胀、纤毛脱落[41]。近江牡蛎的CaHMGB1可以调控LPS诱导的血淋巴细胞的凋亡和坏死[26]。此外发现,栉孔扇贝CfHMGB1重组蛋白可以结合dsDNA[27]。这些研究表明,HMGB1作为贝类重要的炎症介质,通过执行多种功能包括引发炎症反应和启动细胞死亡过程参与机体的抗感染免疫。
1.6 AIF1及其生物学功能
AIF1是一种钙结合蛋白,主要与器官移植排斥、炎性血管病变和自身性免疫疾病等炎性疾病相关。目前,在长牡蛎[28]和近江牡蛎[29]、栉孔扇贝[30]、皱纹盘鲍[31]、菲律宾蛤仔[32]和马氏珠母贝[33]中均鉴定出AIF1(表1),它们均含有两个Ca2+结合的EF-手形结构域,且在多个组织中有表达。大多数贝类的AIF1在血淋巴中的mRNA表达水平相对较高。贝类血淋巴中含有大量行使免疫功能的血淋巴细胞,提示AIF1在贝类固有免疫中发挥重要作用。贝类的AIF1可以响应免疫刺激,在调节贝类免疫应答中发挥重要作用。在LPS、PGN、Poly(I:C)或鳗弧菌刺激后,栉孔扇贝CfAIF1在血淋巴细胞中的mRNA表达水平显著升高,但对Glu无响应[30];在溶藻弧菌、副溶血弧菌(V.parahaemolyticus)或枯草芽孢杆菌(Bacillus subtilis)刺激后,马氏珠母贝PfAIF1在血淋巴细胞中的mRNA表达水平显著升高,且当贝壳或外套膜受到损伤后,血淋巴细胞中PfAIF1的mRNA表达水平也显著升高,提示其在调节贝类组织损伤修复中发挥重要作用[33];长牡蛎CgAIF1重组蛋白可以促进血淋巴细胞中颗粒细胞的吞噬活性,并诱导CgMIF、CgTNF和CgIL17的表达[28];近江牡蛎CaAIF1重组蛋白可以诱导血淋巴细胞发生凋亡和坏死,也可以诱导CaLITAF、CaMyD88和CaTGFβ的表达[29]。这些研究表明,贝类的AIF1可以通过调控细胞免疫和体液免疫参与机体的抗感染免疫过程。
1.7 其他类型炎症细胞因子及其生物学功能
除上述炎症细胞因子外,在贝类中还发现了其他类型的炎症细胞因子,如LITAF和TGFβ。
LITAF是一类保守的细胞因子,在调节LPS诱导的TNF-α的表达过程中起着非常重要的作用。目前,在长牡蛎[34]、马氏珠母贝[35]和皱纹盘鲍[31]中均鉴定出LITAF,它们含有一个LITAF(Zn2+)结合结构域,且在多个组织中表达(表1)。在长牡蛎中发现了6个CgLITAF,它们主要在鳃、消化腺和外套膜中表达[34]。马氏珠母贝PfLITAF在消化腺、鳃和肠中的mRNA表达水平相对较高[35]。皱纹盘鲍HdLITAF在血淋巴细胞中的mRNA表达水平相对较高,其次是闭壳肌和消化腺[31]。贝类LITAF的mRNA可以被多种病原菌诱导。如在长牡蛎中,除CgLITAF4和CgLITAF6外,其他CgLITAF的mRNA在血淋巴细胞中均可以选择性地被不同的PAMP或病原所诱导。其中,CgLITAF2在Poly(I:C)、LPS或溶藻弧菌刺激后的mRNA表达水平显著升高,而CgLITAF3在LPS或PGN刺激后的mRNA表达水平显著升高[34]。
TGFβ是一种在体内广泛分布的多功能细胞因子,在调控细胞增殖、分化、自噬、凋亡、伤口愈合及免疫炎症等方面均发挥重要作用[42-43]。目前,仅在长牡蛎中鉴定出一个TGFβ(CgTGFβ)(表1),其在唇瓣和消化腺中的mRNA表达水平相对较高[36]。在LPS或大肠杆菌刺激后,CgTGFβ在血淋巴细胞中的mRNA表达水平显著升高[36],表明CgTGFβ也可参与长牡蛎的抗感染免疫过程。
2 贝类炎症相关的信号通路
贝类的炎症反应主要是由一系列的炎症相关信号通路所介导,在机体抗病原菌感染中发挥重要作用。贝类炎症相关信号通路主要包括核因子κB(nuclear factor kappa-B,NF-κB)信号通路、MAPK信号通路、信号转导与转录激活因子(signal transducer and activator of transcription,Stat)信号通路、AP-1信号通路和补体系统。这些信号通路最终通过调控多种炎症细胞因子的表达来促进机体的炎症反应,以达到清除病原和维持机体免疫稳态的目的。
2.1 NF-κB信号通路
NF-κB信号通路在调控机体免疫和炎症反应中发挥重要作用。目前,在贝类中也发现了NF-κB信号通路的相关组件,如Toll样受体(Toll-like receptor,TLR)、髓样分化因子(myeloid differentiation factor 88,MyD88)、白介素1受体关联激酶4(interleukin 1 receptor associated kinase 4,IRAK4)、NEMO(NF-κB essential modulator)、IKK1(IκB kinase)、TNF受体相关因子6(TNF receptor associated factor 6,TRAF6)、NF-κB抑制蛋白(inhibitor of NF-κB,IκB)和Rel等(图1)。其中,在长牡蛎中已鉴定出CgTLR、CgMyD88、CgIRAK4、CgNEMO和CgRel等。长牡蛎的CgTLR2和CgTLR6与高等动物的TLR4具有类似的功能,即可以直接结合微生物表面的多糖成分[44-45]。在HEK293T细胞中,CgTLR通过CgMyD88激活NF-κB途径[46]。CgMyD88-1/2可以激活NF-κB途径,而CgMyD88s可以抑制由CgMyD88-1/2激活的NF-κB途径[47]。CgTLR2也可以与CgMyD88-2结合,最终调控炎症细胞因子的表达[45]。在牡蛎疱疹病毒、溶藻弧菌或Poly(I:C)刺激后,CgIRAK4在血淋巴和鳃中的mRNA表达水平显著升高,且CgIRAK4也可以与CgMyD88结合[48]。CgNEMO可以激活CgNF-κB/Rel,促进炎症细胞因子的表达,且在灿烂弧菌刺激后,CgNF-κB/Rel可以进入到血淋巴细胞的细胞核,并诱导多种炎症细胞因子的表达。在栉孔扇贝中已鉴定出CfTLR、CfMyD88、CfNEMO、CfIKK1、CfTRAF6、CfIκB和CfNF-κB[49]。CfTLR可以通过其TIR结构域与CfMyD88结合,CfMyD88又可以与CfIKK1结合,这3个基因在LPS刺激后的mRNA表达水平显著升高[50-51]。CfNEMO可以与CfIKK1结合,且LPS可以诱导CfNF-κB/Rel进入血淋巴细胞的细胞核。在皱纹盘鲍和杂色鲍中也鉴定出TLR(HdTLR)[52]、MyD88s(HdMyD88-2和HdMyD88-X)[53]、IκB(HdIκB和SaIκB)[54]和Rel(HdRel和SaRel)[55-56]。在副溶血弧菌和LPS刺激后,HdTLR在血淋巴和鳃中的mRNA表达水平显著升高。HdMyD88-2和HdMyD88-X均可激活NF-κB途径,并促进一氧化氮、炎症调节因子(iNOS和COX2)和炎症细胞因子(IL1β、IL6和TNFα)的表达[53]。这些研究表明,贝类的NF-κB信号通路在分子组成、激活机制及调控的免疫效应上与高等动物均相似,该通路介导的炎症反应在贝类抗感染免疫中发挥重要作用。

图1 贝类NF-κB信号通路
Fig.1 NF-κB signaling pathway in molluscs
2.2 MAPK信号通路
MAPK信号通路是真核生物信号传递网络中的重要途径之一,在调控细胞增殖、分化和细胞凋亡中发挥重要作用。在贝类中已经鉴定出MAPK信号通路中的关键组件,如MEKK、MKK和MAPK等(图2)。在泥蚶(Tegillarca granosa)中鉴定出TgMEKK4,其mRNA表达水平在副溶血弧菌、溶藻微球菌和LPS刺激后显著升高;将TgMEKK4转染至HEK293T细胞中,发现其可以激活JNK和ERK,但不能激活P38[57]。在虾夷扇贝中鉴定出5个MKKs(PyMKK1/2、PyMKK4、PyMKK5、PyMKK3/6和PyMKK7)[58]。在文蛤中鉴定出MpMAPKK1/4/5/6/7和MpP38,其中,MpMAPKK1/4/5/6含有一个S_TKc结构域,MAPKK7含有一个STYKc结构域[59]。MpMAPK由JNK、ERK和P38组成,主要参与机体的免疫和炎症反应。长牡蛎的CgJNK、CgERK和CgP38广泛分布于多种组织中,且在LPS或灿烂弧菌刺激后,它们在血淋巴细胞中的mRNA表达水平和蛋白磷酸化水平显著升高;进一步研究发现,它们均可以调控CgIL17和CgTNF的表达[60-62]。贝类的MAPK受多条信号通路的调控。如长牡蛎CgERK可以被凝集素CgCLec-HTM和CgSyk激活,活化的CgERK又可促进CgNF-κB/Rel转位入核,最终诱导CgIL17和CgTNF的表达[62]。长牡蛎的CgCLec-TM1识别病原后可以激活ERK,进而诱导多种炎症细胞因子的表达[63]。同时,CgERK可以被含Ig结构域的受体CgIgR激活,活化的CgERK又可促进组蛋白发生甲基化[64]。在灿烂弧菌刺激后,CgBCL10可以激活CgJNK、CgERK和CgP38,进而促进CgNF-κB/Rel转位入核,最终调控CgIL17-1/2/3/6和CgTNF-2的表达[65]。此外发现,炎症细胞因子也可以激活MAPK信号通路。例如,警报素CgHMGB1也可以激活CgERK和CgP38,并诱导CgIL17-5的表达[41]。CgIL17-5又可以激活血淋巴细胞中的CgJNK、CgERK和CgP38,并诱导CgIL17-2/4/6和CgTNF-1的表达[13]。在虾夷扇贝中也鉴定出PyJNK、PyERK和PyP38。鳗弧菌或藤黄微球菌刺激可以促进血淋巴细胞中PyERK和PyJNK的表达,但不诱导PyP38的表达[66]。在缢蛏中也鉴定出一个P38(ScP38)。当敲降ScP38表达后,血淋巴细胞的凋亡率和ROS含量均降低[67]。这些研究表明,贝类MAPK信号通路在调控病原诱导的免疫和炎症反应中发挥重要作用。

图2 贝类MAPK信号通路
Fig.2 MAPK signaling pathway in molluscs
2.3 Stat信号通路
Stat是一条由细胞因子刺激的信号通路,参与细胞增殖、分化、凋亡及免疫调节等多种生物学过程。目前,在马氏珠母贝中也发现了PfJak和PfStat。其中,血淋巴细胞中的PfStat在Poly(I:C)刺激后的mRNA表达水平显著升高[7]。在三角帆蚌中鉴定出3个Stat(HcStat1、HcStat2和HcStat3)。在金黄色葡萄球菌(Staphylococcus aureus)或嗜水气单胞菌(Aeromonas hydrophila)刺激后,这3个基因在血淋巴和肝胰腺中的mRNA表达水平显著升高[68]。在长牡蛎中也发现了该信号通路中的关键因子CgPDGFRβ、CgJak和CgStat。CgPDGFRβ可以结合革兰氏阴性菌和LPS,并发生二聚体,之后与激酶CgSrc结合,促进CgStat进入血淋巴细胞的细胞核,最终诱导CgIL17-4和CgTNF-1的表达[69]。此外,长牡蛎CgSOCS6可以抑制血淋巴细胞中CgStat的转位入核和CgIL17-5、CgIL17-5的表达[70]。这些研究表明,贝类的Stat信号通路可以被不同的病原菌激活,其所介导的炎症反应在贝类抗病原菌感染中发挥重要作用。
2.4 AP-1信号通路
AP-1信号通路可以被多种免疫刺激诱导激活,并参与调节细胞增殖、分化、凋亡及炎症发生等过程。AP-1作为转录因子,主要由Jun和Fos组成。目前,在贝类中仅发现了转录因子Fos,其包含一个Jun结构域和一个bZIP结构域。近江牡蛎ChFos在溶藻弧菌、溶血性葡萄球菌、酿酒酵母菌、LPS、PGN和Poly(I:C)刺激后的mRNA表达水平显著升高,进一步的细胞系过表达试验发现,ChFos主要定位在HEK293T细胞的细胞核中[71]。长牡蛎CgAP-1主要表达于血淋巴细胞的颗粒细胞中。在LPS刺激后,CgAP-1在血淋巴细胞中的mRNA表达水平显著升高。同时,其可以转移到血淋巴细胞的细胞核中,并促进CgIL17-4和CgIL17-5表达[72]。这些研究表明,AP-1信号通路介导的炎症反应在贝类抗感染免疫中发挥重要作用。
2.5 补体系统
补体系统是机体固有免疫的重要组成部分,由40多种血浆蛋白组成,参与对病原微生物的免疫防御。根据补体系统激活过程的不同可以分为经典途径、旁路途径和甘露糖结合凝集素途径。这3条途径激活后均作用于C3,促进C3裂解成C3a和C3b。近年来,在多种贝类中发现了C1q、甘露糖结合凝集素(mannose binding lectin,MBL)、C3、MASP2同源物和B因子[73](图3)。大多数贝类的C1qDC只含有一个或多个C1q结构域,缺失了高等动物C1q所具有的胶原样结构域。它们可以作为PRR,参与病原微生物的识别,并介导血淋巴细胞对微生物的吞噬。近期在长牡蛎中鉴定出一个含有胶原样结构域的C1q分子CgC1qDC6,该分子具有直接识别病原微生物的功能[74]。含有胶原样结构域的C1q分子的发现,提示在贝类中可能存在类经典补体途径。在贝类中也发现了多种类似Ficolin的分子。如在贻贝、牡蛎和扇贝中均发现了FREP分子,它们与脊椎动物中的Ficolin不同,仅包含保守的FBG结构域,缺失了胶原样结构域,但都发挥PRR的功能[73]。在长牡蛎中鉴定出一种与MBL类似的凝集素分子CgCLec-CCP。此外发现,该分子除了含有CRD外,还含有一个补体控制蛋白(complement control protein,CCP)结构域,CgCLec-CCP的CRD具有直接识别病原微生物和多糖的功能[75]。同时,从牡蛎、缢蛏、三角帆蚌和贻贝中鉴定出C3样分子,它们含有与哺乳动物C3相似的保守结构域。在贝类中也筛选出多个MASP,它们均含有多个CUB和一个丝氨酸蛋白酶结构域,但缺失了两个CCP结构域。B因子作为替代途径的关键分子,目前在贝类中也有报道[76-77]。如在缢蛏中鉴定出一个ScBf分子,该分子包含两个CCP结构域、一个VWA结构域和一个丝氨酸蛋白酶结构域。进一步研究发现,ScBf具有凝集兔红细胞的功能[76]。在贝类中除了鉴定出补体系统的关键元件外,还解析了一条凝集素途径[75]。在长牡蛎中,CgCLec-CCP可以与CgMASPL-1相互作用,活化的CgMASPL-1又可与CgC3结合并诱导CgC3的裂解[73,75]。贝类补体系统最终可以介导多种免疫效应,如溶菌活性、炎症反应、细胞自噬和细胞吞噬等(图3)。以上研究表明,在贝类中已经进化出了凝集素途径,且可能存在经典途径和替代途径。
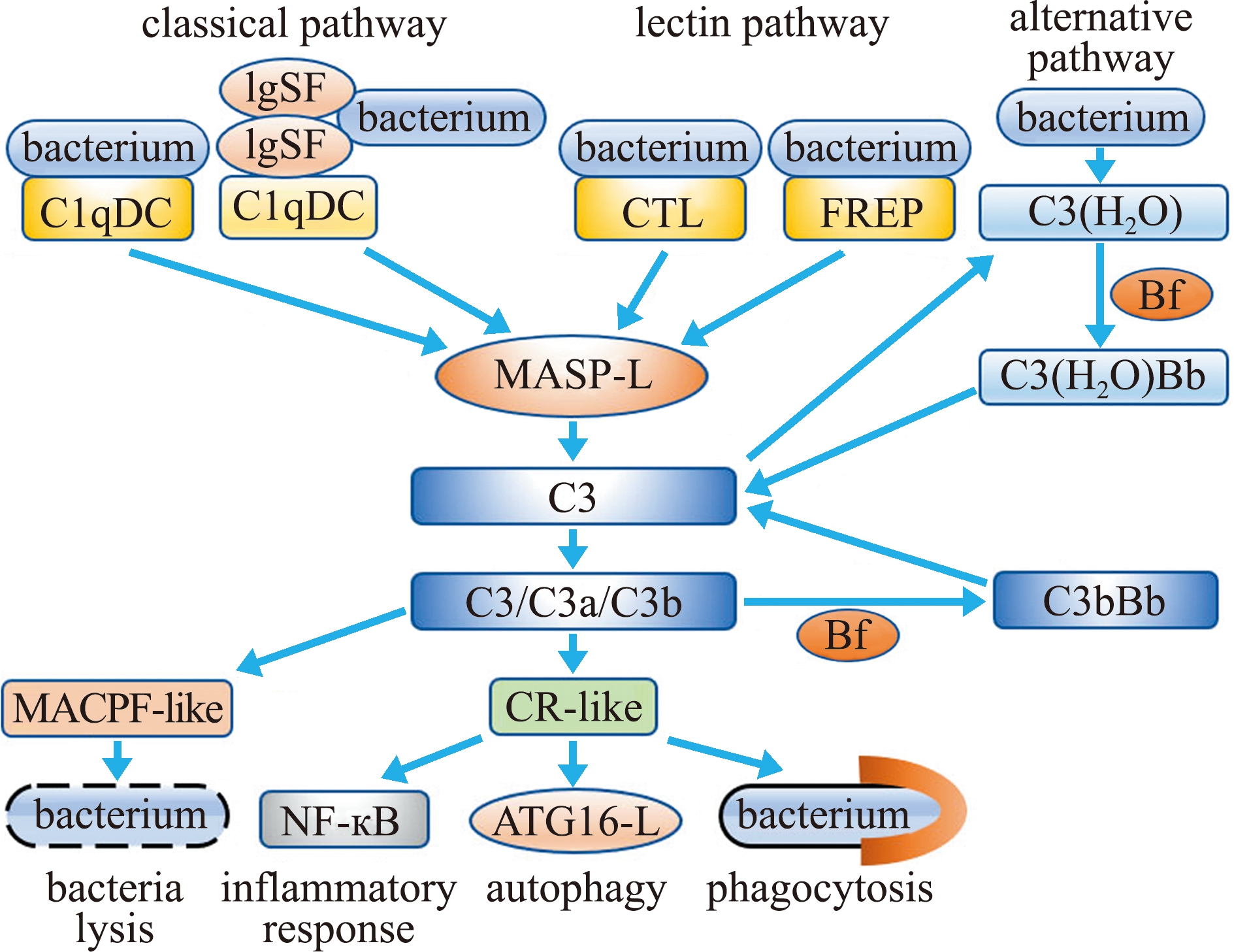
图3 贝类补体系统
Fig.3 Complement system in molluscs
3 贝类炎症反应的形态学特征和诱发因素
病原侵入后引发的炎症反应是宿主组织的局部防御反应。在高等动物中,炎症反应可以通过局部循环(如充血和血管通透性增加)募集免疫细胞(如粒细胞、淋巴细胞和巨噬细胞)到损伤病灶,以清除病原和损伤细胞,并最终启动组织修复过程[78-79]。在贝类中,炎症反应根据其形态特征可分为浸润型、结节型和包裹型[80]。浸润型炎症反应的特征是免疫细胞对损伤组织的局部或弥漫性侵袭。如在Steinhausia mytilovum和Marteilia refringens感染的贻贝中,观察到严重的弥漫性炎症现象[80-81]。结节型炎症反应的特征是观察到大量小颗粒被吞噬和免疫细胞聚集,并在循环系统和间隙组织中形成由小到大的团簇[80,82]。结节主要由层状的免疫细胞包膜包裹着退化的免疫细胞、外来颗粒和其他碎片组成[81]。在贝类中,结节形成通常被认为是病原菌感染导致的免疫细胞聚集的结果[83]。由活的和坏死的血淋巴细胞聚集在病变组织中的现象常被称为脓肿[81,84]。如感染哈维弧菌的鲍,足部肌肉经常观察到脓肿现象[80]。包裹型反应是一种常见的针对异物的免疫防御反应,这些异物如多细胞寄生虫因个体太大而无法被血淋巴细胞吞噬[80-81]。
诱发贝类产生炎症反应的因素有很多,主要包括细菌、病毒和寄生虫感染等。如灿烂弧菌感染的贝类鳃出现鳃丝肿胀、纤毛脱落、血淋巴细胞浸润和细胞坏死等现象;溶藻弧菌感染的贻贝肝胰腺出现血淋巴细胞增生,组织核肿大,并伴有细胞坏死[85];疱疹病毒OsHV-1感染的贝类也出现不同程度的组织病理变化,包括鳃丝毛细血管腔内出现大量血淋巴细胞聚集,外套膜结缔组织中的典型结构消失,且在肝胰腺的消化小管与肠道周边观察到大量血淋巴细胞浸润现象[86];鲍疱疹病毒感染的鲍神经节出现细胞坏死与神经胶质细胞增殖,同时肝胰腺组织出现细胞坏死[87];帕金虫感染的蛤仔,部分鳃丝上皮细胞发生核固缩,鳃纤毛脱落,结缔组织中的嗜酸性颗粒细胞发生聚集[88];复殖吸虫感染的蛤仔,性腺组织遭到严重破坏,视野下无精卵细胞存在[89]。此外,一些物理因素也可以导致贝类组织发生炎症反应。例如,高温胁迫可以导致长牡蛎鳃出现鳃丝肿胀、纤毛脱落、血淋巴细胞浸润和结缔组织不规则等现象;重金属胁迫也可以引起长牡蛎部分组织出现不同程度的病理变化(待发表)。
4 存在问题及展望
炎症反应在生物体响应环境胁迫、抗感染免疫及维持内环境稳态中均发挥重要作用。贝类种类繁多,生活环境复杂,适应能力强,进化地位特殊,是研究炎症起源与进化的重要类群。目前已发现,在贝类中存在炎症细胞因子、炎症相关信号通路和炎症反应,研究其间的相互关系,对于了解和认识炎症的起源和进化具有重要意义。尽管在贝类中已经发现了部分炎症细胞因子和炎症相关信号通路,但对其他类型炎症细胞因子的挖掘及贝类炎症细胞因子在调节机体免疫应答中的作用机制仍然缺乏系统深入的认识,未来仍需在以下方面重点开展研究。
4.1 进一步挖掘贝类中潜在的炎症细胞因子
在贝类中,尽管已经发现了部分IL,但对除IL17和IL12之外的其他类型的IL样分子仍然未知。在贝类中已经鉴定出一个类似IFN的分子,但关于该分子在抗病毒中的作用及是否存在不同类型的IFN仍需进一步探究。此外,在贝类中有关趋化因子的研究仍是空白。贝类种类繁多,但有关炎症细胞因子的研究主要集中在少数种类中。通过挖掘贝类中存在的不同类型的炎症细胞因子,可为进一步了解和认识炎症细胞因子的起源和进化提供重要线索。
4.2 揭示不同类型炎症细胞因子在调控贝类免疫应答中的作用
炎症细胞因子在调控机体免疫应答中发挥重要作用。在贝类中尽管已经鉴定出部分类型的炎症细胞因子,但有关它们结合的膜受体及其介导的下游免疫信号通路和免疫效应仍需深入探究。同时,不同类型的炎症细胞因子之间存在的相互关系及其在调控免疫应答中发挥的协同/拮抗作用仍需深入系统地研究。
4.3 阐明贝类炎症细胞因子与不同类型免疫细胞之间的关系
贝类血淋巴细胞主要包括3种类型(透明细胞、半透明细胞和颗粒细胞),是机体主要行使免疫功能的细胞。不同的炎症细胞因子由不同类型的免疫细胞产生,所产生的炎症细胞因子又通过自分泌或旁分泌方式发挥作用。然而,在贝类中有关炎症细胞因子的产生机制及产生后所作用的细胞类型有待深入探索。
[1] NEURATH M F.Cytokines in inflammatory bowel disease[J].Nature Reviews Immunology,2014,14(5):329-342.
[2] AMARASEKARA D S,YUN H,KIM S,et al.Regulation of osteoclast differentiation by cytokine networks[J].Immune Network,2018,18(1):e8.
[3] WANG L L,SONG X R,SONG L S.The oyster immunity[J].Developmental &Comparative Immunology,2018,80:99-118.
[4] XIN L S,LIU C,ZHANG H,et al.The characterization of an interleukin-12 p35 homolog involved in the immune modulation of oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2021,123:104145.
[5] WU S Z,HUANG X D,LI Q,et al.Interleukin-17 in pearl oyster (Pinctada fucata):molecular cloning and functional characterization[J].Fish &Shellfish Immunology,2013,34(5):1050-1056.
[6] LI J,ZHANG Y,ZHANG Y H,et al.Genomic characterization and expression analysis of five novel IL-17 genes in the Pacific oyster,Crassostrea gigas[J].Fish &Shellfish Immunology,2014,40(2):455-465.
[7] HUANG X D,ZHANG H A,HE M X.Comparative and evolutionary analysis of the interleukin 17 gene family in invertebrates[J].PLoS One,2015,10(7):e0132802.
[8] SACO A,REY-CAMPOS M,ROSANI U,et al.The evolution and diversity of interleukin-17 highlight an expansion in marine invertebrates and its conserved role in mucosal immunity[J].Frontiers in Immunology,2021,12:692997.
[9] ZHANG R,WANG M,XIA N,et al.Cloning and analysis of gene expression of interleukin-17 homolog in triangle-shell pearl mussel,Hyriopsis cumingii,during pearl sac formation[J].Fish &Shellfish Immunology,2016,52:151-156.
[10] ROSANI U,VAROTTO L,GERDOL M,et al.IL-17 signaling components in bivalves:comparative sequence analysis and involvement in the immune responses[J].Developmental &Comparative Immunology,2015,52(2):255-268.
[11] CAO W Q,WANG W L,FAN S Q,et al.The receptor CgIL-17R1 expressed in granulocytes mediates the CgIL-17 induced haemocytes proliferation in Crassostrea gigas[J].Developmental &Comparative Immunology,2022,131:104376.
[12] XIN L S,ZHANG H,ZHANG R,et al.CgIL17-5,an ancient inflammatory cytokine in Crassostrea gigas exhibiting the heterogeneity functions compared with vertebrate interleukin 17 molecules[J].Developmental &Comparative Immunology,2015,53(2):339-348.
[13] LV X Q,SUN J J,LI Y N,et al.CgIL17-5 regulates the mRNA expressions of immune effectors through inducing the phosphorylation of CgMAPKs and the nuclear translocation of CgRel and CgAP-1 in the Pacific oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2022,127:104263.
[14] SUN Y,ZHOU Z,WANG L L,et al.The immunomodulation of a novel tumor necrosis factor (CgTNF-1) in oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2014,45(2):291-299.
[15] ZHENG Y,LIU Z Q,WANG L L,et al.A novel tumor necrosis factor in the Pacific oyster Crassostrea gigas mediates the antibacterial response by triggering the synthesis of lysozyme and nitric oxide[J].Fish &Shellfish Immunology,2020,98:334-341.
[16] DE ZOYSA M,JUNG S,LEE J.First molluscan TNF-α homologue of the TNF superfamily in disk abalone:molecular characterization and expression analysis[J].Fish &Shellfish Immunology,2009,26(4):625-631.
[17] MART N-G
N-G MEZ L,VILLALBA A,CARBALLAL M J,et al.Molecular characterisation of TNF,AIF,dermatopontin and VAMP genes of the flat oyster Ostrea edulis and analysis of their modulation by diseases[J].Gene,2014,533(1):208-217.
MEZ L,VILLALBA A,CARBALLAL M J,et al.Molecular characterisation of TNF,AIF,dermatopontin and VAMP genes of the flat oyster Ostrea edulis and analysis of their modulation by diseases[J].Gene,2014,533(1):208-217.
[18] ZHANG R,LIU R,WANG W L,et al.Identification and functional analysis of a novel IFN-like protein (CgIFNLP) in Crassostrea gigas[J].Fish &Shellfish Immunology,2015,44(2):547-554.
[19] LI F M,HUANG S Y,WANG L L,et al.A macrophage migration inhibitory factor like gene from scallop Chlamys farreri:involvement in immune response and wound healing[J].Developmental &Comparative Immunology,2011,35(1):62-71.
[20] PARISI M G,TOUBIANA M,MANGANO V,et al.MIF from mussel:coding sequence,phylogeny,polymorphism,3D model and regulation of expression[J].Developmental &Comparative Immunology,2012,36(4):688-696.
[21] CUI S G,ZHANG D C,JIANG S G,et al.A macrophage migration inhibitory factor like oxidoreductase from pearl oyster Pinctada fucata involved in innate immune responses[J].Fish &Shellfish Immunology,2011,31(2):173-181.
[22] WANG D,YANG D L,WANG Q,et al.Two macrophage migration inhibitory factors (MIFs) from the clam Ruditapes philippinarum:molecular characterization,localization and enzymatic activities[J].Fish &Shellfish Immunology,2018,78:158-168.
[23] WANG B Z,ZHANG Z P,WANG Y L,et al.Molecular cloning and characterization of macrophage migration inhibitory factor from small abalone Haliotis diversicolor supertexta[J].Fish &Shellfish Immunology,2009,27(1):57-64.
[24] ZOU L H,LIU B Z.The polymorphisms of a MIF gene and their association with Vibrio resistance in the clam Meretrix meretrix[J].Developmental &Comparative Immunology,2016,62:116-126.
[25] LI J,ZHANG Y,XIANG Z M,et al.High mobility group box 1 can enhance NF-κB activation and act as a pro-inflammatory molecule in the Pacific oyster,Crassostrea gigas[J].Fish &Shellfish Immunology,2013,35(1):63-70.
[26] XU T,YANG S B,XIE J S,et al.HMGB in mollusk Crassostrea ariakensis gould:structure,pro-inflammatory cytokine function characterization and anti-infection role of its antibody[J].PLoS One,2012,7(11):e50789.
[27] WANG M Q,WANG L L,GUO Y,et al.A high mobility group box 1 (HMGB1) gene from Chlamys farreri and the DNA-binding ability and pro-inflammatory activity of its recombinant protein[J].Fish &Shellfish Immunology,2014,36(2):393-400.
[28] ZHANG Y,LI J,YU F,et al.Allograft inflammatory factor-1 stimulates hemocyte immune activation by enhancing phagocytosis and expression of inflammatory cytokines in Crassostrea gigas[J].Fish &Shellfish Immunology,2013,34(5):1071-1077.
[29] XU T,XIE J S,ZHU B J,et al.Allograft inflammatory factor 1 functions as a pro-inflammatory cytokine in the oyster,Crassostrea ariakensis[J].PLoS One,2014,9(4):e95859.
[30] WANG J J,ZHANG H,WANG L L,et al.Molecular cloning and transcriptional regulation of an allograft inflammatory factor-1 (AIF-1) in Zhikong scallop Chlamys farreri[J].Gene,2013,530(2):178-184.
[31] DE ZOYSA M,NIKAPITIYA C,KIM Y,et al.Allograft inflammatory factor-1 in disk abalone (Haliotis discus discus):molecular cloning,transcriptional regulation against immune challenge and tissue injury[J].Fish &Shellfish Immunology,2010,29(2):319-326.
[32] ZHANG L,ZHAO J M,LI C H,et al.Cloning and characterization of allograft inflammatory factor-1 (AIF-1) from Manila clam Venerupis philippinarum[J].Fish &Shellfish Immunology,2011,30(1):148-153.
[33] LI J,CHEN J H,ZHANG Y,et al.Expression of allograft inflammatory factor-1 (AIF-1) in response to bacterial challenge and tissue injury in the pearl oyster,Pinctada martensii[J].Fish &Shellfish Immunology,2013,34(1):365-371.
[34] YU F,ZHANG Y,YU Z N.Characteristics and expression patterns of the lipopolysaccharide-induced TNF-α factor (LITAF) gene family in the Pacific oyster,Crassostrea gigas[J].Fish &Shellfish Immunology,2012,33(4):899-908.
[35] ZHANG D C,JIANG J J,JIANG S G,et al.Molecular characterization and expression analysis of a putative LPS-induced TNF-α factor (LITAF) from pearl oyster Pinctada fucata[J].Fish &Shellfish Immunology,2009,27(3):391-396.
[36] LELONG C,BADARIOTTI F,LE QU R
R H,et al.Cg-TGF-β,a TGF-β/activin homologue in the Pacific oyster Crassostrea gigas,is involved in immunity against gram-negative microbial infection[J].Developmental &Comparative Immunology,2007,31(1):30-38.
H,et al.Cg-TGF-β,a TGF-β/activin homologue in the Pacific oyster Crassostrea gigas,is involved in immunity against gram-negative microbial infection[J].Developmental &Comparative Immunology,2007,31(1):30-38.
[37] WU W,SUN J J,DONG M R,et al.CgTNF-2 promotes the proliferation of haemocytes by regulating the expressions of CgRunx and cell cycle related genes in the Pacific oyster Crassostrea gigas[J].Fish &Shellfish Immunology,2023,132:108478.
[38] GAO D H,QIU L M,GAO Q,et al.Repertoire and evolution of TNF superfamily in Crassostrea gigas:implications for expansion and diversification of this superfamily in Mollusca[J].Developmental &Comparative Immunology,2015,51(2):251-260.
[39] QIAO X,LI Y J,JIN Y H,et al.The involvement of an interferon-induced protein 44-like (CgIFI44L) in the antiviral immune response of Crassostrea gigas[J].Fish &Shellfish Immunology,2022,129:96-105.
[40] LI Y M,QIAO X,LIU Z Q,et al.A myxovirus resistance like protein involved in CgIFNLP mediated immune response of oyster Crassostrea gigas[J].Fish &Shellfish Immunology,2021,119:318-328.
[41] LV X Q,YANG W W,GUO Z C,et al.CgHMGB1 functions as a broad-spectrum recognition molecule to induce the expressions of CgIL17-5 and Cgdefh2 via MAPK or NF-κB signaling pathway in Crassostrea gigas[J].International Journal of Biological Macromolecules,2022,211:289-300.
[42] KELLY A,HOUSTON S A,SHERWOOD E,et al.Regulation of innate and adaptive immunity by TGFβ[M]//Advances in Immunology.Amsterdam:Elsevier,2017:137-233.
[43] TRAVIS M A,SHEPPARD D.TGF-β activation and function in immunity[J].Annual Review of Immunology,2014,32:51-82.
[44] WANG W L,ZHANG T,WANG L L,et al.A new non-phagocytic TLR6 with broad recognition ligands from Pacific oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2016,65:182-190.
[45] FAN S Q,WANG W L,LI J L,et al.The truncated MyD88s negatively regulates TLR2 signal on expression of IL17-1 in oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2022,133:104446.
[46] ZHANG Y,HE X C,YU F,et al.Characteristic and functional analysis of toll-like receptors (TLRs) in the lophotrocozoan,Crassostrea gigas,reveals ancient origin of TLR-mediated innate immunity[J].PLoS One,2013,8(10):e76464.
[47] TANG X Y,HUANG B Y,LIN S H,et al.CgMyD88s serves as an innate immune system plug during ostreid herpesvirus 1 infection in the Pacific oyster (Crassostrea gigas)[J].Frontiers in Immunology,2020,11:1247.
[48] TANG X Y,HUANG B Y,ZHANG L L,et al.Molecular characterization of Pacific oyster (Crassostrea gigas) IRAK4 gene and its role in MyD88-dependent pathway[J].Developmental &Comparative Immunology,2017,72:21-29.
[49] WANG M Q,YANG J L,ZHOU Z,et al.A primitive Toll-like receptor signaling pathway in mollusk Zhikong scallop Chlamys farreri[J].Developmental &Comparative Immunology,2011,35(4):511-520.
[50] QIU L M,SONG L S,YU Y D,et al.Identification and characterization of a myeloid differentiation factor 88 (MyD88) cDNA from Zhikong scallop Chlamys farreri[J].Fish &Shellfish Immunology,2007,23(3):614-623.
[51] LI L L,LIU W J,FAN N N,et al.Scallop IKK1 responds to bacterial and virus-related pathogen stimulation and interacts with MyD88 adaptor of toll-like receptor pathway signaling[J].Frontiers in Immunology,2022,13:869845.
[52] ELVITIGALA D A S,PREMACHANDRA H K A,WHANG I,et al.Molecular insights of the first gastropod TLR counterpart from disk abalone (Haliotis discus discus),revealing its transcriptional modulation under pathogenic stress[J].Fish &Shellfish Immunology,2013,35(2):334-342.
[53] PRIYATHILAKA T T,BATHIGE S D N K,LEE S,et al.Molecular identification and functional analysis of two variants of myeloid differentiation factor 88 (MyD88) from disk abalone (Haliotis discus discus)[J].Developmental &Comparative Immunology,2018,79:113-127.
[54] KASTHURI S R,WHANG I,NAVANEETHAIYER U,et al.Molecular characterization and expression analysis of IκB from Haliotis discus discus[J].Fish &Shellfish Immunology,2013,34(6):1596-1604.
[55] DE ZOYSA M,NIKAPITIYA C,OH C,et al.Molecular evidence for the existence of lipopolysaccharide-induced TNF-α factor (LITAF) and Rel/NF-kB pathways in disk abalone (Haliotis discus discus)[J].Fish &Shellfish Immunology,2010,28(5/6):754-763.
[56] ZHANG X,HUANG Y T,CAI X H,et al.Identification and expression analysis of immune-related genes linked to Rel/NF-κB signaling pathway under stresses and bacterial challenge from the small abalone Haliotis diversicolor[J].Fish &Shellfish Immunology,2014,41(2):200-208.
[57] LIU G S,CHEN M L,YU C,et al.Molecular cloning,characterization and functional analysis of a putative mitogen-activated protein kinase kinase kinase 4 (MEKK4) from blood clam Tegillarca granosa[J].Fish &Shellfish Immunology,2017,66:372-381.
[58] ZOU J J,WANG R J,LI R J,et al.The genome-wide identification of mitogen-activated protein kinase kinase (MKK) genes in Yesso scallop Patinopecten yessoensis and their expression responses to bacteria challenges[J].Fish &Shellfish Immunology,2015,45(2):901-911.
[59] ZHANG S J, YU J J, WANG H X, et al. p38 MAPK is involved in the immune response to pathogenic Vibrio in the clam Meretrix petechialis[J].Fish &shellfish immunology, 2019(95): 456-463.
[60] SUN J J,WANG L L,WU Z J,et al.P38 is involved in immune response by regulating inflammatory cytokine expressions in the Pacific oyster Crassostrea gigas[J].Developmental &Comparative Immunology,2019,91:108-114.
[61] SUN J J,LI Y N,LI M J,et al.A novel JNK is involved in immune response by regulating IL expression in oyster Crassostrea gigas[J].Fish &Shellfish Immunology,2018,79:93-101.
[62] SUN J J,WANG L L,HUANG M M,et al.CgCLec-HTM-mediated signaling pathway regulates lipopolysaccharide-induced CgIL-17 and CgTNF production in oyster[J].The Journal of Immunology,2019,203(7):1845-1856.
[63] SUN J J,GAO L,HUANG S,et al.CLec-TM1-ERK-GSK3β pathway regulates Vibrio splendidus-induced IL-17 production in oyster[J].The Journal of Immunology,2021,207(2):640-650.
[64] SUN J J,WANG L L,YANG C Y,et al.An ancient BCR-like signaling promotes ICP production and Hemocyte phagocytosis in oyster[J].iScience,2020,23(2):100834.
[65] ZHANG T,SUN J J,WANG L Y,et al.BCL10 regulates the production of proinflammatory cytokines by activating MAPK-NF-κB/Rel signaling pathway in oysters[J].Fish &Shellfish Immunology,2022,120:369-376.
[66] SUN Y,ZHANG L L,ZHANG M W,et al.Characterization of three mitogen-activated protein kinases (MAPK) genes reveals involvement of ERK and JNK,not p38 in defense against bacterial infection in Yesso scallop Patinopecten yessoensis[J].Fish &Shellfish Immunology,2016,54:507-515.
[67] WANG Y Z,HAN Y T,WANG Y H,et al.Expression of p38MAPK and its regulation of apoptosis under high temperature stress in the razor clam Sinonovacula constricta[J].Fish &Shellfish Immunology,2022,122:288-297.
[68] DAI Y J,HUI K M,ZHANG Y H,et al.Three STATs are involved in the regulation of the expression of antimicrobial peptides in the triangle sail mussel,Hyriopsis cumingii[J].Fish &Shellfish Immunology,2017,63:181-188.
[69] SUN J J,WU Z J,WU W,et al.PDGFRβ recognizes and binds bacteria to activate src/stat pathway in oysters[J].The Journal of Immunology,2021,207(12):3060-3069.
[70] WU Z J,SUN J J,WANG L Y,et al.CgSOCS6 negatively regulates the expression of CgIL17s and CgDefh1 in the Pacific oyster Crassostrea gigas[J].Fish &Shellfish Immunology,2019,93:1084-1092.
[71] QU F F,XIANG Z M,WANG F X,et al.A novel molluscan Fos gene with immune defense function identified in the Hong Kong oyster,Crassostrea hongkongensis[J].Developmental &Comparative Immunology,2015,51(1):194-201.
[72] WANG L Y,SUN J J,WU Z J,et al.AP-1 regulates the expression of IL17-4 and IL17-5 in the Pacific oyster Crassostrea gigas[J].Fish &Shellfish Immunology,2020,97:554-563.
[73] SUN J J,WANG L L,SONG L S.The primitive complement system in molluscs[J].Developmental &Comparative Immunology,2023,139:104565.
[74] LI H,KONG N,SUN J J,et al.A C1qDC (CgC1qDC-6) with a collagen-like domain mediates hemocyte phagocytosis and migration in oysters[J].Developmental &Comparative Immunology,2019,98:157-165.
[75] SUN J J,WANG L Y,YANG W W,et al.A novel C-type lectin activates the complement cascade in the primitive oyster Crassostrea gigas[J].Journal of Biological Chemistry,2021,297(6):101352.
[76] PENG M X,LI Z,NIU D H,et al.Complement factor B/C2 in molluscs regulates agglutination and illuminates evolution of the Bf/C2 family[J].The FASEB Journal,2019,33(12):13323-13333.
[77] PRADO-ALVAREZ M,ROTLLANT J,GESTAL C,et al.Characterization of a C3 and a factor B-like in the carpet-shell clam,Ruditapes decussatus[J].Fish &Shellfish Immunology,2009,26(2):305-315.
[78] CONE J B.Inflammation[J].The American Journal of Surgery,2001,182(6):558-562.
[79] MULLER W A.Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response[J].Trends in Immunology,2003,24(6):326-333.
[80] DE VICO G,CARELLA F.Morphological features of the inflammatory response in molluscs[J].Research in Veterinary Science,2012,93(3):1109-1115.
[81] GALLOWAY T S,DEPLEDGE M H.Immunotoxicity in invertebrates:measurement and ecotoxicological relevance[J].Ecotoxicology,2001,10(1):5-23.
[82] CARELLA F,ACETO S,MAIOLINO P,et al.What is your diagnosis?Pale yellowish digestive gland and watery tissues in Mediterranean mussels[J].Veterinary Clinical Pathology,2011,40(2):273-274.
[83] ROWLEY A F.The evolution of inflammatory mediators[J].Mediators of Inflammation,1996,5(1):3-13.
[84] HINE P M.Southern hemisphere mollusc diseases and an overview of associated risk assessment problems[J].Revue Scientifique et Technique,1996,15(2):563-577.
[85] LAITH A A,ROS-AMIRA M K,SHEIKH H I,et al.Histopathological and immunological changes in green mussel,Perna viridis, challenged with Vibrio alginolyticus[J].Fish &Shellfish Immunology,2021,118:169-179.
[86] BURIOLI E A V,VARELLO K,LAVAZZA A,et al.A novel divergent group of Ostreid herpesvirus 1 muVar variants associated with a mortality event in Pacific oyster spat in Normandy (France) in 2016[J].Journal of Fish Diseases,2018,41(11):1759-1769.
[87] CORBEIL S,COLLING A,WILLIAMS L M,et al.Development and validation of a TaqMan PCR assay for the Australian abalone herpes-like virus[J].Diseases of Aquatic Organisms,2010,92(1):1-10.
[88] 梁玉波.菲律宾蛤仔体内寄生帕金虫的研究[D].青岛:中国科学院研究生院(海洋研究所),2005.
LIANG Y B.Study on endoparasite Perkinsns sp.in the Manila clams Ruditapes philippinarum in China[D].Qingdao:Graduate University,Chinese Academy of Sciences(Institute of Oceanography),2005.(in Chinese)
[89] 孟祥宇.感染复殖吸虫菲律宾蛤仔Ruditapes philippinarum转录组学分析[D].大连:大连海洋大学,2018.
MENG X Y.Study on transcriptome of Manila clam(Ruditapes philippinarum) infected by digenetic trematode[D].Dalian:Dalian Ocean University,2018.(in Chinese)