5-羟色胺(5-hydroxy tryptamine,5-HT)最早由Rapport于1941年在血清中发现并分离出来,故又名血清素(serotonin),是目前被研究最多的神经递质。鱼类5-HT会对包括攻击行为、情绪调节、自发游泳活动、食物摄入和应激反应等在内的一系列行为和生理产生影响[1-2]。鱼体在应激源的刺激下,会出现适应性的生理变化,诱导5-HT的释放和转换,5-HT经相应受体介导后,参与调控动物的应激、内分泌和情绪反应等一系列行为表现和生理过程[3-4]。环境友好和生产过程可控的设施养殖模式符合“创新、协调、绿色、开放、共享”的发展理念,是水产养殖发展的重要方向[5-6]。一直以来,设施养殖的优化主要集中在改进设施设备和提高管理策略方面,对鱼类行为本身的关注较少。随着精细化装备和鱼类福利水平要求的提高,针对养殖环境下,如何批量筛选特定行为和生理优势的群体,逐渐成为近年来研究的热点[7-8]。5-HT作为调节鱼类行为最重要的神经递质类物质之一,了解5-HT及其受体如何调控鱼类行为和生理过程,有助于揭示不同养殖环境下鱼类的行为和生理适应策略,可为智能化装备研发和精细管理技术的提高提供理论依据和数据支撑[9-10]。本文中以5-HT及其受体为对象,对其调控鱼类行为和生理研究进展进行了综述,以期为鱼类行为和生理响应及养殖环境下提高鱼类福利水平研究提供参考。
1 5-HT及其受体
5-HT的前体是色氨酸(tryptophan,Trp),经色氨酸羟化酶(tryptophan hydroxylase,TPH)作用形成5-羟色氨酸(5-hydroxy tryptophan,5-HTP),再经氨基酸脱羧酶(amino acid decarboxylase,AADC)催化生成5-HT,然后在单胺氧化酶(monoamine oxidase,MAO)作用下氧化为5-羟基-3-乙醛(5-hydroxyindole-3-acetaldehyde,5-HIAL),最后在乙醛脱氢酶(acetaldehyde dehydrogenase,ALDH)作用下脱氢成5-羟吲哚乙酸(5-hydroxyindole acetic acid,5-HIAA),最终随尿液排出体外(图1)。5-HT广泛存在于动物的大脑和肠道神经元等多种外周组织中[11],在硬骨鱼中,脑5-HT主要分布在后脑中缝核外;外周组织中的5-HT主要储存于囊泡内,是由消化系统中的胃肠道嗜铬细胞在胞质内经过一系列酶促反应后生成的。5-HT经5-HT能神经元释放后,通过改变突触间隙中5-HT的含量,完成中枢神经系统的信号传递[12],从而对周围细胞产生广泛而系统的影响。通过阻断5-HT在突触间的再摄取,如药物注射选择性5-HT再摄取抑制剂(SSRIs),能够提高中枢神经系统5-HT含量,抑制神经突触细胞对神经递质5-HT的再吸收,以增加细胞外与突触后受体结合的5-HT含量,缓解与抑郁、焦虑相关的反应[13]。SSRIs的作用在鱼类研究中已经被证明[14],常用的SSRIs包括氟西汀(fluoxetine)、帕罗西汀(paroxetine)、氟伏沙明(fluvoxamine)和舍曲林(sertraline)等。
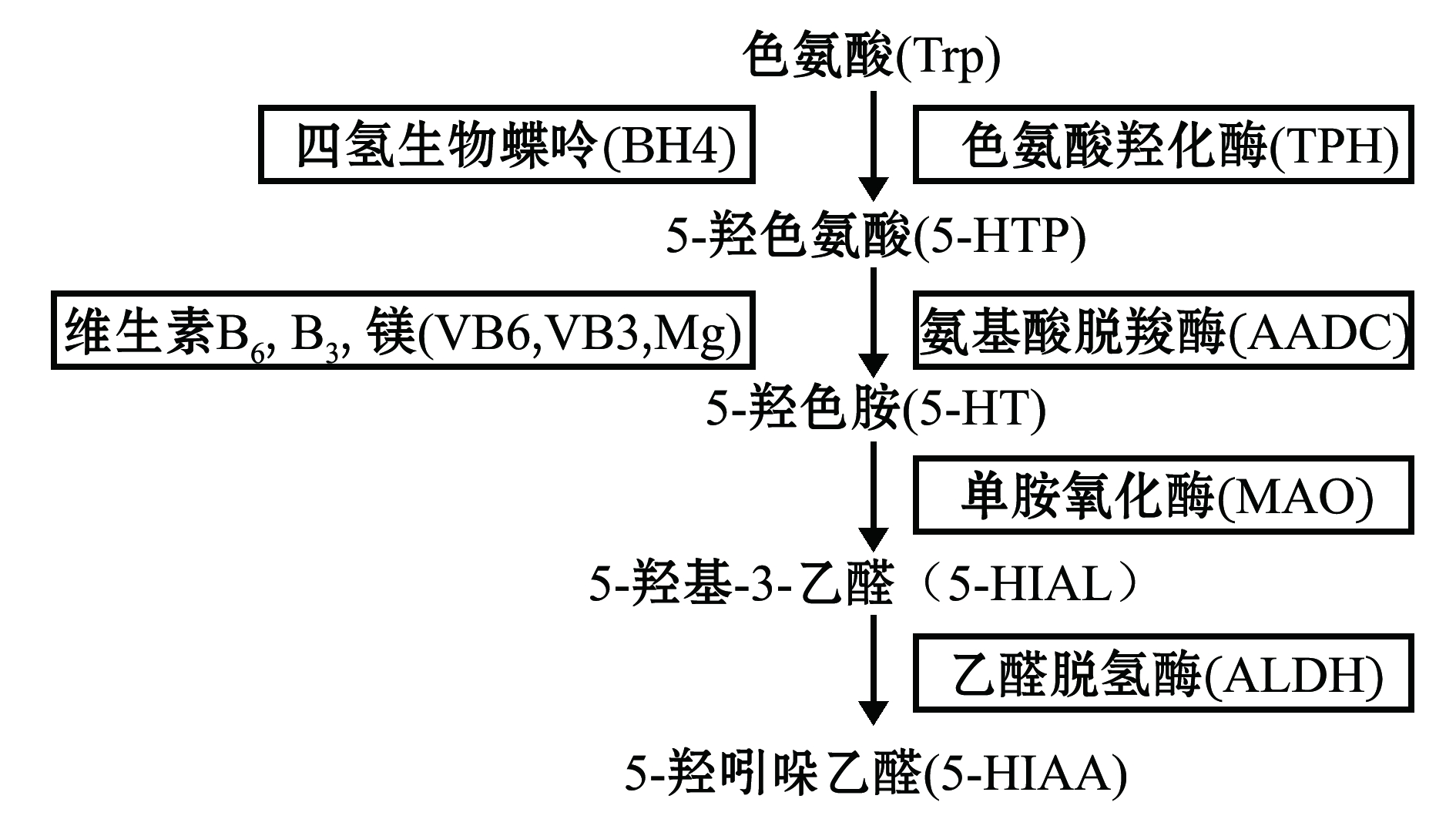
图1 5-HT的代谢过程
Fig.1 Metabolic process of 5-HT
对5-HT的深入研究表明,5-HT必须与受体结合才能发挥作用[15]。迄今为止,在对哺乳动物5-HT受体的研究中共发现7个亚科(5-HT1~5-HT7)共16种5-HT受体亚型,硬骨鱼中共发现11种受体亚型(表1)。除5-HT3受体属于配体门控离子通道超家族(ligand-gated ion channels,LGIC)外,其余5-HT受体都属于G蛋白偶联受体超家族(G Protein-Coupled Receptors,GPCRs)。除此之外,硬骨鱼类5-HT受体存在多个同源基因编码特定受体亚型的现象。如硬骨鱼5-HT1A受体存在2种旁基因,即5-HT1Aα和5-HT1Aβ受体且这两种旁基因具有不同的表达。5-HT受体参与下的5-HT释放或再吸收可能引起多种药理作用。5-HT受体的多样性导致了5-HT神经递质系统的复杂性,目前,已有靶向特定5-HT受体亚型的选择性激动剂和拮抗剂相继被开发(表1),用于探测相应受体的定位、信号特性和中枢神经系统功能。
表1 硬骨鱼中的5-HT受体亚型及相应激动剂和拮抗剂
Tab.1 Receptors and corresponding agonists and antagonists of 5-HT in fish
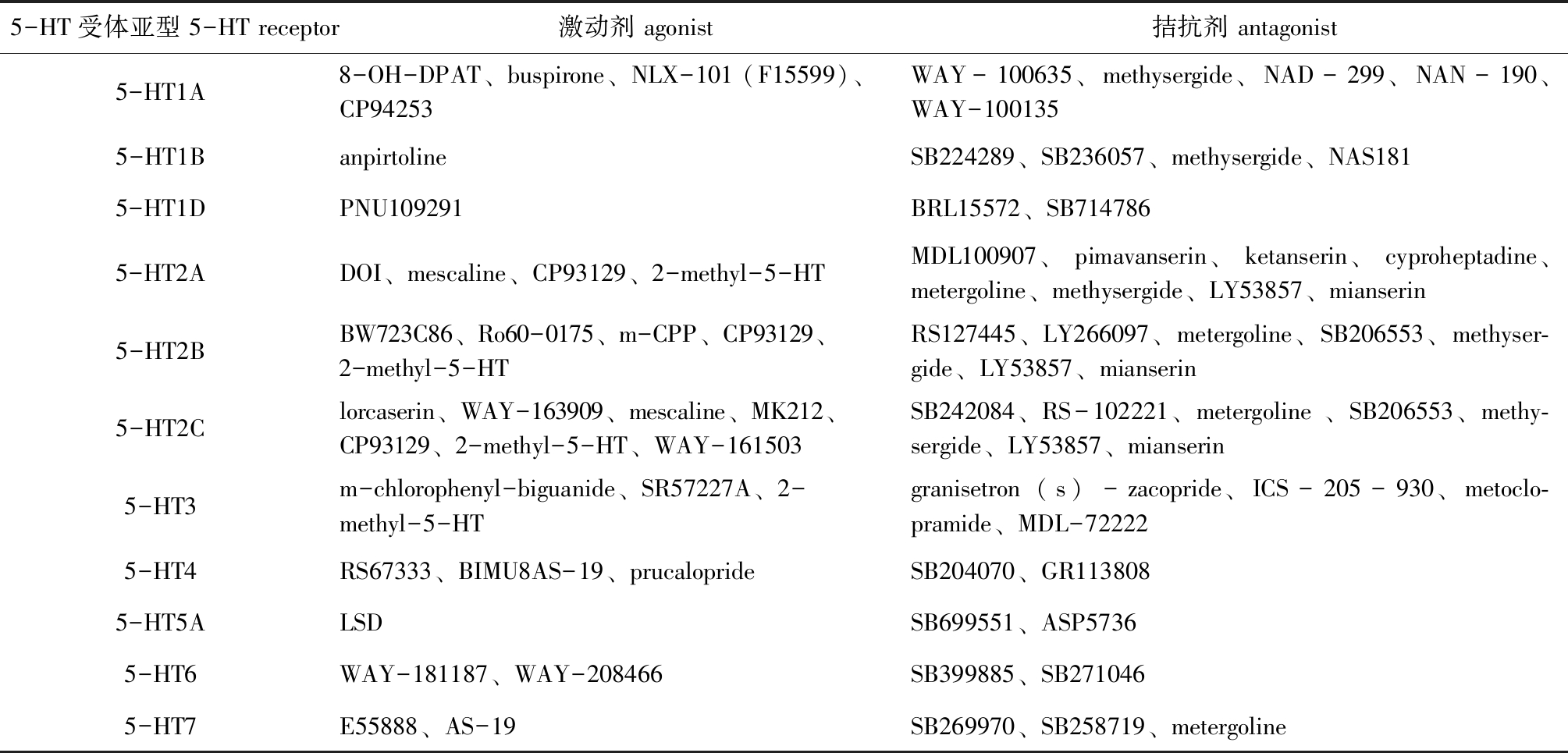
5-HT受体亚型 5-HT receptor激动剂 agonist拮抗剂 antagonist5-HT1A8-OH-DPAT、buspirone、NLX-101 (F15599)、CP94253WAY-100635、methysergide、NAD-299、NAN-190、WAY-1001355-HT1BanpirtolineSB224289、SB236057、methysergide、NAS1815-HT1DPNU109291BRL15572、SB7147865-HT2ADOI、mescaline、CP93129、2-methyl-5-HTMDL100907、pimavanserin、ketanserin、cyproheptadine、metergoline、methysergide、LY53857、mianserin5-HT2BBW723C86、Ro60-0175、m-CPP、CP93129、2-methyl-5-HTRS127445、LY266097、metergoline、SB206553、methyser-gide、LY53857、mianserin5-HT2Clorcaserin、WAY-163909、mescaline、MK212、CP93129、2-methyl-5-HT、WAY-161503SB242084、RS-102221、metergoline 、SB206553、methy-sergide、LY53857、mianserin5-HT3m-chlorophenyl-biguanide、SR57227A、2-methyl-5-HTgranisetron (s)-zacopride、ICS-205-930、metoclo-pramide、MDL-722225-HT4RS67333、BIMU8AS-19、prucaloprideSB204070、GR1138085-HT5ALSDSB699551、ASP57365-HT6WAY-181187、WAY-208466SB399885、SB2710465-HT7E55888、AS-19SB269970、SB258719、metergoline
2 5-HT及其受体对鱼类行为的影响
2.1 攻击行为
攻击行为是一种受多因素交互影响和作用的复杂社会行为[16]。5-HT能够显著影响鱼类攻击行为的表现,随着5-HT水平的降低,鱼类一般会表现出攻击性提高的现象[17]。对虹鳟Oncorhynchus mykiss攻击行为的研究表明,在饲料中添加5-HT的前体L-色氨酸,虹鳟第一次攻击潜伏期的时间出现延长,攻击次数出现显著降低[18]。对玉丽体鱼Cichlasoma dimerus的研究表明,饲料中添加L-色氨酸降低了雄性的攻击动机和显性攻击行为,从而间接影响了劣势鱼的竞争性行为,这些行为结果可能是大脑5-HT功能的改变所引起的[19]。双带锦鱼Thalassoma bifasciatum注射fluoxetine后,表现出活动水平减少,对入侵鱼的追逐和攻击频率下降等现象[20]。对斑马鱼Danio rerio攻击行为的研究表明,经fluoxetine暴露2 h处理后,优势斑马鱼的攻击次数和追逐时间显著下降,劣势斑马鱼的静止时间也出现显著下降[21]。
5-HT受体调控鱼类攻击行为的研究主要集中在5-HT1和5-HT2受体上。对五彩搏鱼Betta splendens的研究表明,5-HT1A受体激动剂8-OH-DPAT可有效降低其攻击强度[22],对奥马罗裸背电鳗Gymnotus omarorum的研究也得到同样的结果[23]。相似地,斑马鱼经过5-HT1A受体拮抗剂WAY-100635处理后,表现出更显著地攻击性[24]。但五彩搏鱼经腹腔注射5-HT1A受体拮抗剂WAY-100635后,其攻击性并未表现出显著性差异[22],造成这种结果的原因可能是由于试验设置(外源药物介入的方式、预处理的时间或药物使用的剂量)不同所引起的。同一群体中的海湾豹蟾鱼Opsanus beta在经fluoxetine处理后,优势鱼的攻击性显著增加,但劣势鱼的攻击水平在处理前后无显著性差异,优势和劣势鱼脑中5-HT1A受体的mRNA表达均无显著性差异[25]。上述结果表明,5-HT不同受体之间的功能并不是完全独立的,除5-HT1A受体外,其他受体类型也可能共同参与鱼类攻击行为的调控。对斑马鱼攻击性的研究表明,斑马鱼在接受fluoxetine处理后,脑组织中的5-HT1B mRNA水平出现显著上调[21]。除5-HT1受体外,5-HT2受体也可能发挥相似的作用。在形成稳定社会等级的伯氏妊丽鱼Astatotilapia burtoni群体中,处于劣势地位的低攻击雄鱼端脑中的5-HT1A和5-HT2A受体基因表达量均显著高于高攻击个体[26],这说明5-HT1A和5-HT2A受体的功能存在相似性。
2.2 社会等级
鱼类社会等级是指同种鱼类会在群体内逐渐建立一种优势-劣势(或支配-从属)社会地位的差异关系,是鱼类群体行为最重要的属性之一[27-28]。鱼类社会等级的建立、发展和稳定与5-HT系统密切相关[1,17],研究表明,在一定程度上调控5-HT水平可以逆转鱼群已经形成的稳定的社会等级[29]。对大西洋鲑Salmo salar幼鱼的研究发现,无论优势鱼还是劣势鱼,均表现为体内5-HTAA和5-HTAA/5-HT水平的增加,不同的是优势鱼中这两者水平会迅速下降,调整至平均水平,而在劣势鱼中,这两者的含量则会在相对较长的一段时间内保持较高水平[1]。随着社会等级的稳定,劣势鱼某些行为会表现出被抑制的倾向,如被频繁攻击、摄食减少和运动力下降等[30-31]。许氏平鮋Sebastes schlegelii在社会等级建立初期,劣势鱼后端脑的5-HIAA/5-HT比值显著高于优势鱼,随着社会等级的稳定,劣势鱼所有脑区的5-HIAA/5-HT比值均显著高于优势鱼[17]。
5-HT受体主要通过影响肾上腺皮质激素和皮质醇等激素的释放和抑制,进而参与调控下丘脑-垂体-肾间质轴(HPI轴),实现对鱼类社会等级的调节[32-33]。5-HT受体对社会等级的调控主要体现在5-HT1受体上。5-HT1A受体的激活,可以显著增加海湾豹蟾鱼下丘脑区域垂体促肾上腺皮质激素(adrenocorticotropic hormone,ACTH)的分泌和促肾上腺皮质激素释放因子(corticotropin releasing factor,CRF)前体mRNA的表达[34]。5-HT1A受体除了可以刺激促肾上腺皮质激素的分泌外,也会刺激皮质醇的释放,而皮质醇是表征鱼类应激反应的主要指标之一,其水平代表了鱼体的应激状况,继而对个体的社会等级产生影响[35-36]。研究表明,虹鳟的血浆皮质醇水平随着5-HT1A受体激动剂8-OH-DPAT剂量浓度的升高而升高[37];皮质醇的升高导致海湾豹蟾鱼下丘脑5-HT1A mRNA表达下调,而注射5-HT1A激动剂8-OH-DPAT则会抑制皮质醇的分泌[38]。以上研究表明,5-HT1A受体参与介导了皮质醇的分泌,进一步证明了硬骨鱼中5-HT1A受体和皮质醇之间的相关性。此外,5-HT1A受体可能在下丘脑和垂体水平上发挥作用,并通过刺激激素的释放参与鱼类社会等级形成。如虹鳟5-HT4 受体通过头肾组织激活HPI轴,刺激HPI轴的表达[39],但HPI轴是否是通过5-HT4受体来调控鱼类社会等级,还需进一步研究。
2.3 情绪调节
鱼具有感知痛苦的能力,随着鱼类福利日益受到重视,关于5-HT受体在鱼类情绪调节中的作用研究也逐渐成为热点[40]。斑马鱼经5-HT1A受体激动剂buspirone处理后,在光亮处停留的时间显著高于未经药物处理组[41],同样的研究结果也在斑马鱼注射5-HT1A受体激动剂8-OH-DPAT的研究中被报道,这表明,5-HT1A受体的激活对斑马鱼能够起到显著的抗焦虑或镇静作用[42]。此外,对同一个卵巢内不同时期出生的大西洋鲑进行焦虑应激试验,结果显示,后期出生的大西洋鲑5-HT1A受体的mRNA表达升高,而早期出生的大西洋鲑没有此现象,其行为表现更大胆和更具攻击性,这说明大西洋鲑 5-HT1A受体对鱼类抗焦虑的作用可能与鱼类的生活史特征有关[43]。除5-HT1受体外,5-HT2受体同样能够对鱼类的情绪调节产生作用[44-45]。斑马鱼在注射5-HT1和5-HT2受体拮抗剂methysergide后,处于高浓度预警物质中的恐惧反应显著增加,表现为出现大量的冻结行为、在水槽底部时间增加和游动减少等行为[46]。相反,斑马鱼经5-HT2A/2C受体激动剂mescaline处理后,表现为集群行为的显著增加和运动模式的改变(如探索行为减少和明显的抗焦虑行为)[47],这说明5-HT2A/2C受体的激活可降低斑马鱼的焦虑反应。值得一提的是,对伯氏妊丽鱼社会等级的研究发现,在调节鱼类惊吓行为的M细胞中发现了5-HT5A受体亚型和5-HT6受体,这说明5-HT5A受体亚型和5-HT6受体可能参与了鱼类的情绪反应和调节[48]。
2.4 学习认知能力
5-HT系统在哺乳动物认知障碍或发病治疗中发挥着重要作用[49],但5-HT及其受体对鱼类学习认知能力的研究相对较少。最近的研究表明,鱼类可能通过5-HT1A受体的介导来实现学习记忆和社会选择。对金鱼Carassius auratus学习主动回避刺激的研究发现,与对照组相比,经5-HT1A受体拮抗剂WAY-100635处理后的金鱼表现出更多主动回避刺激的行为[50],这说明5-HT1A受体对金鱼的认知能力有积极作用。经5-HT1A受体激动剂buspirone处理后,对集群无偏好的斑马鱼表现为群体内相互距离的减小和集群时间的增加[51],这表明5-HT1A受体可能增强了鱼类之间的交流能力。除5-HT1A受体外,哺乳动物中5-HT6受体也被证实与学习认知和长期记忆功能有关,而斑马鱼5-HT6受体与人类5-HT6受体具有高度同源性[52],这提示5-HT6受体也可能参与调控鱼类的学习认知能力。
5-HT系统在调节鱼类社会互动中同样起着重要作用。对裂唇鱼Labroides dimidiatus合作行为的研究发现,经药物注射fluoxetine的裂唇鱼个体能够为客户鱼提供更多触觉刺激,提高了参与清洁互动的频率,但经5-HT1A受体拮抗剂WAY-10065处理后并没有类似的效果[53],这说明5-HT1A受体对鱼类社会互动的调节作用较小。对孔雀鱼Poecilia reticulata在应对捕食者时的合作行为研究表明,fluoxetine处理后孔雀鱼观察捕食者图像的时间增加,躲避捕食者的时间减少,表明5-HT水平的增加促进了“条件接近”合作策略的形成(第一个个体先接近危险物,其他个体只有跟随先接近个体游泳时,才会靠近危险物)[54]。但经5-HT2和5-HT7受体拮抗剂metergoline处理后,孔雀鱼观察捕食者的时间显著减少,表明5-HT2和5-HT7受体可能参与了调控鱼类社会合作,但主要的受体作用类型和机制尚不明确。
3 5-HT及其受体对鱼类生理的影响
3.1 摄食
5-HT通过控制摄食神经网络进而参与调控鱼类摄食。鱼类正常摄食时,5-HT代谢处于正常水平;摄食量增加时,5-HT代谢会增加,但并不影响脑内糖原水平;摄食量减少时,5-HT通过参与调控脑内糖原的分解,释放能量以满足鱼类紧急状态下的生理需求[55]。对虹鳟自需摄食的研究表明,有触发记录个体的5-HIAA/5-HT水平显著高于无触发个体[56];对饥饿胁迫条件下虹鳟的研究结果表明,虹鳟的脑糖原水平随着饥饿时间的延长存在依赖性下降,在饥饿状态基础上对侧脑室和腹腔注射5-HT后,虹鳟脑中糖原水平降低更为显著[55],这说明5-HT代谢与鱼类摄食显著相关。
5-HT通过受体介导实现对摄食量的调控,同时摄食量的变化也会影响5-HT受体的表达。腹腔注射5-HT1A受体激动剂8-OH-DPAT后,虹鳟摄食被显著抑制;脑内注射5-HT2C受体激动剂MK212后,虹鳟摄食同样被显著抑制;但注射5-HT2B受体激动剂BW723C86后,虹鳟的摄食表现与对照组相比无显著性差异[57]。5-HT2C受体对食物摄入量的影响主要是作用于神经肽,通过增加鱼类下丘脑阿黑皮质素(pro-opiomelanocortin,POMC)、促肾上腺皮质激素释放因子和苯丙胺调节转录肽(cocaine and amphetamine-regulated transcript,CART)的分泌和释放来实现[58]。同时,摄食量的变化也会引起5-HT受体表达的变化。当鱼类摄食量发生变化时,5-HT受体也会做出相应的调整。摄食量过少的虹鳟经5-HT1A、5-HT1B和5-HT2受体激动剂(8-OH-DPAT、anpirtoline、CP93129和2-methyl-5-HT)处理后,其脑中的糖原水平显著下降;而经5-HT1A、5-HT1B和5-HT2B/C受体拮抗剂(WAY-100135、NAN190、NAS181和SB206553)的处理可以拮抗这种作用,但5-HT2受体的激动和拮抗作用程度比在5-HT1受体中观察到的要小[55]。以上结果表明,5-HT1A、5-HT1B和5-HT2B/C受体均可能参与调节鱼类摄食过少时的糖原分解过程,并且5-HT1A和5-HT1B介导的作用似乎更强。
外周组织中5-HT主要存在于肠道的上皮细胞中,5-HT能够通过调控肠道活动和消化功能来参与调控鱼类摄食行为[59]。位于胆碱能神经元的5-HT4和5-HT7受体参与调控了金鱼前肠的收缩活动[60]。5-HT4受体能够促进胚胎后期肠神经发生的发育,经5-HT4受体激动剂prucalopride处理后的斑马鱼,其神经发生损伤组的肠神经元再生加快,同时神经发生未损伤组的后肠收缩活动显著增加[61]。以上结果表明,5-HT4和5-HT7受体可能通过调控肠内神经元促进肠道运动,控制鱼类食物摄入,从而实现对鱼类摄食的调控。
3.2 免疫
在哺乳动物中,5-HT不能穿过血脑屏障(blood brain barrier,BBB),但硬骨鱼类的5-HT是可以通过血脑屏障的[62],因此,5-HT在鱼类外周组织中也可能会达到较高水平,并且影响中枢5-HT的变化[63]。外周循环5-HT最显著的作用是调节鱼体的免疫系统,对5-HT受体影响鱼类免疫系统的研究主要集中在5-HT1、5-HT2和5-HT3受体上,但受体发挥的作用和功能不同。
与鱼类免疫系统相关的5-HT1受体主要为5-HT1A和5-HT2A受体。5-HT1A受体通过参与淋巴细胞功能的表达来调控鱼类免疫功能。对蓝鳃太阳鱼Lepomis macrochirus的研究表明,5-HT1A受体激动剂8-OH-DPAT通过作用于脾细胞,能够显著抑制鱼体淋巴细胞的增殖[64];对虹鳟注射5-HT1A受体激动剂8-OH-DPAT也得到相似的结果,同时研究还指出,5-HT1受体拮抗剂spiperone可以逆转8-OH-DPAT诱导的这种抑制[65]。值得一提的是,在静息细胞中,5-HT1A受体激动剂(8-OH-DPAT和buspirone)和拮抗剂NAN-190对 3H-5HT与受体位点的结合无显著性影响,但这两者在有丝分裂(脂多糖LPS和植物凝集素PHA)刺激的淋巴细胞中能够显著抑制 3H-5HT的结合,这表明5-HT1A受体在鱼类静息淋巴细胞中不表达,必须在有丝分裂的刺激下才能表达,并与有丝分裂原(LPS和PHA)的抑制反应有关[65]。
对海湾豹蟾鱼尿素脉动排泄的研究发现,5-HT2A受体的mRNA表达量在鱼鳔和性腺中最高,5-HT2A受体拮抗剂ketanserin的作用会导致自发性尿素排泄的脉动成分显著下降,能够调节鱼类脉动性的尿素排泄,这表明5-HT2A受体在鱼体内是通过其独特的鸟氨酸循环(尿素循环)来促进鱼体毒素排出的[66]。这些结果表明,5-HT2A受体可能是通过调节尿素排泄等其他行为过程从而参与鱼类体内免疫反应的调控。
5-HT3受体是5-HT受体的一种特殊类型,属于配体门控离子通道超家族,5-HT3受体通过结合向内和向外的免疫活性细胞中的离子,从而影响鱼类免疫细胞功能[67-68]。虹鳟经5-HT3受体激动剂2-methyl-5-HT处理后,其外周淋巴细胞中Ca+含量显著增加,5-HT3受体介导了硬骨鱼类外周淋巴细胞中的Ca+从细胞外环境中的调动,5-HT3受体激动剂2-methyl-5-HT刺激了Na+的向内运动,而拮抗剂ICS-205-930、metoclopramide和MDL-72222则显著抑制了这种现象[69],证明Na+是通过5-HT3受体通道进入鱼类淋巴细胞中。此外,5-HT3受体激动剂2-methyl-5-HT对受有丝分裂原刺激的鱼类T淋巴细胞具有剂量依赖的免疫抑制作用;而拮抗剂ICS-205-930和metoclopramide可以部分逆转这种作用,这表明鱼类5-HT3受体在T细胞增殖中也发挥了重要作用[69]。
3.3 生殖
5-HT通过对下丘脑-垂体-性腺轴(HPG轴)的调控,参与调节鱼类的生殖功能和过程。对成年雄性纹眼笛鲷Lutjanus argentiventris的繁殖行为研究表明,其端脑的5-HT水平在产卵前达到峰值,在产卵期间最低[70]。对大西洋黄鱼Micropogonias undultus的研究发现,5-HT2受体拮抗剂LY53857几乎可以完全消除5-HT刺激诱导促性腺激素水平(GtH Ⅱ)的分泌作用,5-HT2受体激动剂DOI则增强了这种作用[71]。对金鱼的研究表明,5-HT1A激动剂8-OH-DPAT和5-HT2受体激动剂2-methyl-5-HT 也会抑制促性腺激素水平的释放,而经5-HT2A受体拮抗剂ketanserin和cyproheptadine的处理,可阻断鱼类促性腺激素的释放[72],这表明5-HT1A和5-HT2受体可能共同参与了鱼类促性腺激素的分泌和鱼类生殖功能的调控。除此之外,阻断5-HT合成,会改变罗非鱼Oreochromis mossambicus性别分化关键期脑芳香化酶的活性[73],这表明5-HT还可能影响鱼类的性别分化过程。5-HT受体还会对生长激素(growth hormone,GH)的调控产生作用。金鱼5-HT1A受体激动剂8-OH-DPAT可以刺激GH的释放,但5-HT2受体激动剂2-methyl-5-HT能够抑制基础GH的分泌,同时拮抗剂mianserin会阻断这种抑制作用,这表明5-HT对基础GH释放的抑制仅通过5-HT2受体介导,5-HT1受体并不参与这种抑制作用[72]。
4 存在问题及展望
4.1 5-HT及其受体对鱼类行为和生理影响研究中存在的问题
随着养殖智能化的快速发展,未来对鱼类行为学的研究需求将快速上升,作为最主要的神经化学因子之一,5-HT对鱼类行为和生理的影响研究应用前景广阔。随着技术的发展和研究的深入,近年来关于5-HT对鱼类行为的作用表现和调控机制正在得到逐步揭示。然而,在基础研究和相关产业中的进一步应用还需解决以下几个方面的问题:
1)鱼类5-HT受体研究的种类有待进一步扩大。由于5-HT1、5-HT2受体对5-HT的亲和力高于其他5-HT受体,因此,现阶段对鱼类5-HT及其受体的研究多集中这两种受体上,对鱼类中大多数受体(5-HT3~5-HT7)的研究较为缺乏。
2)5-HT及其受体对鱼类行为和生理的调控机制尚不明确。目前,5-HT及其受体对鱼类行为和生理的调控作用已经被证实,但是对其调控机制的研究主要集中在鱼类5-HT受体基因与啮齿动物、人类的同源性分析和不同受体亚型在不同组织的分布方面,相关的分子生物学机制尚未明确。
3)5-HT受体亚型之间的耦合/拮抗作用还有待进一步研究。5-HT不同受体之间的功能并不是完全独立的,不同受体亚型在鱼类各行为中所起的作用、重要程度及靶向性等问题还有待深入研究。
4)对5-HT及其受体如何调节养殖鱼类行为和生理影响的研究较少。与医学和神经发生学相比,对5-HT及其受体在鱼类上的研究,主要集中在斑马鱼等模式生物上,缺乏针对养殖鱼类的研究,研究内容也较为局限,多集中在繁育和摄食等方面,从鱼类福利水平出发的相关研究较少。
4.2 未来重点研究方向
对5-HT及其受体调控鱼类行为和生理的深入研究,将使人们更为深入地了解有关鱼类自适应能力的基本规律,同时也将在优化和繁育上产生新的应用前景。作者针对目前研究中存在的问题,提出了在鱼类5-HT及其受体研究领域有待深入开展的研究方向有以下几方面:
1)5-HT不同受体类型基本功能的研究。在调控鱼类行为的多种5-HT受体类型中,起关键作用的受体类型尚不明确。除此之外,不同受体类型对鱼类行为和生理的调控作用方式是相对独立的还是具有交互作用尚不明确,若存在交互,为耦合还是拮抗。因此,亟须了解5-HT不同受体类型在鱼类行为和生理上的基本功能。
2)5-HT作用机制的研究。在鱼类行为和生理的自适应过程中,5-HT作为一种调节因子参与了其他神经因子的合成与释放,虽然行为的物质基础主要是神经系统,但神经系统最终形成的生理生化性能是受遗传物质基因控制的,未来的研究需要进一步阐明行为、中枢神经系统及其相关基因(基因表达)之间的作用机制,以明确5-HT合成、储存、膜摄取和代谢的具体调控通路和过程。
3)5-HT在鱼类行为可塑性上的研究应用。鱼类行为是内在条件和外在环境综合作用的结果,在养殖背景下,应加强行为生态学研究,扩大5-HT系统在鱼类行为可塑性研究方面的应用,特别是明确不同养殖应激条件下,5-HT系统的表现差异与鱼类行为、生理的耦合关系,进一步细化5-HT系统在调控鱼类不同行为上的作用,减少养殖条件胁迫,从而提高水产养殖效率。
4)5-HT及其受体对鱼类行为及其生理响应的调控。根据养殖对象的适应程度来优化养殖环境和批量筛选群体内适合度高的个体,并以此提供解决途径,为养殖智能化水平的提高和鱼类福利关注度的提升提供理论依据和数据支撑。
[1] WINBERG S,NILSSON G E.Roles of brain monoamine neurotransmitters in agonistic behaviour and stress reactions,with particular reference to fish[J].Comparative Biochemistry and Physiology Part C:Pharmacology Toxicology and Endocrinology,1993,106(3):597-614.
[2] 文竹,李强,杜亚松,等.发育早期暴露氟西汀对斑马鱼社交行为发展的影响[J].中国儿童保健杂志,2015,23(12):1267-1271.
WEN Z,LI Q,DU Y S,et al.Effects on long-lasting social-related behavior in early postnatal fluoxetine treated on zebrafish[J].Chinese Journal of Child Health Care,2015,23(12):1267-1271.(in Chinese)
[3] PUGLISI-ALLEGRA S,ANDOLINA D.Serotonin and stress coping[J].Behavioural Brain Research,2015,277:58-67.
[4] BACKSTRÖM T,WINBERG S.Serotonin coordinates responses to social stress-what we can learn from fish[J].Frontiers in Neuroscience,2017,11:595.
[5] 孙国祥.大西洋鲑工业化循环水养殖投喂策略研究[D].青岛:中国科学院研究生院(海洋研究所),2014.
SUN G X.Feeding strategy study for Atlantic salmon (Salmo salar L.)in recirculating aquaculture systems[D].Qingdao:Graduate University of the Chinese Academy of Sciences(Institute of Oceanology),2014.(in Chinese)
[6] MEZZOMO N J,MÜLLER T E,FRANSCESCON F,et al.Taurine-mediated aggression is abolished via 5-HT1A antagonism and serotonin depletion in zebrafish[J].Pharmacology Biochemistry and Behavior,2020,199:173067.
[7] CASTANHEIRA M F,CONCEIÇ O L E C,MILLOT S,et al.Coping styles in farmed fish:consequences for aquaculture[J].Reviews in Aquaculture,2017,9(1):23-41.
O L E C,MILLOT S,et al.Coping styles in farmed fish:consequences for aquaculture[J].Reviews in Aquaculture,2017,9(1):23-41.
[8] ZHAO D,FENG P.Temperature increase impacts personality traits in aquatic non-native species:implications for biological invasion under climate change[J].Current Zoology,2015,61:966-971.
[9] BARCELLOS H H A,KOAKOSKI G,CHAULET F,et al.The effects of auditory enrichment on zebrafish behavior and physiology[J].Peer J,2018,6:e5162.
[10] COX K,BRENNAN L P,GERWING T G,et al.Sound the alarm:a meta-analysis on the effect of aquatic noise on fish behavior and physiology[J].Global Change Biology,2018,24(7):3105-3116.
[11] FOSSAT P,BACQUÉ-CAZENAVE J,DE DEURWAERDER P,et al.Anxiety-like behavior in crayfish is controlled by serotonin[J].Science,2014,344(6189):1293-1297.
[12] DE-MIGUE F F,TRUETA C.Synaptic and extrasynaptic secretion of serotonin[J].Cellular and Molecular Neurobiology,2005,25(2):297-312.
[13] MCDONALD M D.An AOP analysis of selective serotonin reuptake inhibitors (SSRIs)for fish[J].Comparative Biochemistry and Physiology Part C:Toxicology and Pharmacology,2017,197:19-31.
[14] VALENTI JR T W,GOULD G G,BERNINGER J P,et al.Human therapeutic plasma levels of the selective serotonin reuptake inhibitor (SSRI)sertraline decrease serotonin reuptake transporter binding and shelter-seeking behavior in adult male fathead minnows[J].Environmental Science and Technology,2012,46(4):2427-2435.
[15] SHARP T,BARNES N M.Central 5-HT receptors and their function:present and future[J].Neuropharmacology,2020,177:108-155.
[16] SUMMERS C H,FORSTER G L,KORZAN W J,et al.Dynamics and mechanics of social rank reversal[J].Journal of Comparative Physiology A,2005,191(3):241-252.
[17] XU X,ZHANG Z,GUO H,et al.Changes in aggressive behavior,cortisol and brain monoamines during the formation of social hierarchy in black rockfish (Sebastes schlegelii)[J].Animals,2020,10(12):2357.
[18] WINBERG S,ØVERLI Ø,LEPAGE O.Suppression of aggression in rainbow trout (Oncorhynchus mykiss)by dietary L-tryptophan[J].Journal of Experimental Biology,2001,204(22):3867-3876.
[19] MORANDINI L,RAMALLO M R,SCAIA M F,et al.Dietary l-tryptophan modulates agonistic behavior and brain serotonin in male dyadic contests of a cichlid fish[J].Journal of Comparative Physiology A,2019,205(6):867-880.
[20] PERREAULT H A N,SEMSAR K,GODWIN J.Fluoxetine treatment decreases territorial aggression in a coral reef fish[J].Physiology and Behavior,2003,79(4/5):719-724.
[21] THEODORIDI A,TSALAFOUTA A,PAVLIDIS M.Acute exposure to fluoxetine alters aggressive behavior of zebrafish and expression of genes involved in serotonergic system regulation[J].Frontiers in Neuroscience,2017,11:223.
[22] CLOTFELTER E D,O’HARE E P,MCNITT M M,et al.Serotonin decreases aggression via 5-HT1A receptors in the fighting fish Betta splendens[J].Pharmacology Biochemistry and Behavior,2007,87(2):222-231.
[23] ZUBIZARRETA L,PERRONE R,STODDARD P K,et al.Differential serotonergic modulation of two types of aggression in weakly electric fish[J].Frontiers in Behavioral Neuroscience,2012,6:77.
[24] FILBY A L,PAULL G C,HICKMORE T F A,et al.Unravelling the neurophysiological basis of aggression in a fish model[J].BMC Genomics,2010,11(1):1-17.
[25] MCDONALD M D,GONZALEZ A,SLOMAN K A.Higher levels of aggression are observed in socially dominant toadfish treated with the selective serotonin reuptake inhibitor,fluoxetine[J].Comparative Biochemistry and Physiology Part C:Toxicology and Pharmacology,2011,153(1):107-112.
[26] LOVELAND J L,UY N,MARUSKA K P,et al.Social status differences regulate the serotonergic system of a cichlid fish,Astatotilapia burtoni[J].Journal of Experimental Biology,2014,217(15):2680-2690.
[27] CAMMARATA M,VAZZANA M,ACCARDI D,et al.Seabream (Sparus aurata)long-term dominant-subordinate interplay affects phagocytosis by peritoneal cavity cells[J].Brain Behavior and Immunity,2012,26(4):580-587.
[28] AKBARIPASAND A,KRKOSEK M,LOKMAN P M,et al.Does social status within a dominance hierarchy mediate individual growth,residency and relocation?[J].Oecologia,2014,176(3):771-779.
[29] SUMMERS C H,KORZAN W J,LUKKES J L,et al.Does serotonin influence aggression?Comparing regional activity before and during social interaction[J].Physiological and Biochemical Zoology,2005,78(5):679-694.
[30] SUMMERS C H,LARSON E T,SUMMERS T R,et al.Regional and temporal separation of serotonergic activity mediating social stress[J].Neuroscience,1998,87(2):489-496.
[31] ØVERLI Ø,HARRIS C A,WINBERG S.Short-term effects of fights for social dominance and the establishment of dominant-subordinate relationships on brain monoamines and cortisol in rainbow trout[J].Brain Behavior and Evolution,1999,54(5):263-275.
[32] EJIKE C,SCHRECK C B.Stress and social hierarchy rank in Coho salmon[J].Transactions of The American Fisheries Society,1980,109(4):423-426.
[33] HÖGLUND E,BALM P H M,WINBERG S.Stimulatory and inhibitory effects of 5-HT1A receptors on adrenocorticotropic hormone and cortisol secretion in a teleost fish,the Arctic charr (Salvelinus alpinus)[J].Neuroscience Letters,2002,324(3):193-196.
[34] MEDEIROSL R,CARTOLANO M C,MCDONALD M D.Crowding stress inhibits serotonin 1A receptor-mediated increases in corticotropin-releasing factor mRNA expression and adrenocorticotropin hormone secretion in the Gulf toadfish[J].Journal of Comparative Physiology B,2014,184(2):259-271.
[35] POTTINGER T G,CARRICK T R.Stress responsiveness affects dominant-subordinate relationships in rainbow trout[J].Hormones and Behavior,2001,40(3):419-427.
[36] AIDOS M L,CAFISO A,SERRA V,et al.How different stocking densities affect growth and stress status of Acipenser baerii early stage larvae[J].Animals,2020,10(8):1289.
[37] WINBERG S,NILSSON A,HYLLAND P,et al.Serotonin as a regulator of hypothalamic-pituitary-interrenal activity in teleost fish[J].Neuroence Letters,1997,230(2):113-116.
[38] MEDEIROS L R,MCDONALD M D.Cortisol-mediated downregulation of the serotonin 1A receptor subtype in the Gulf toadfish,Opsanus beta[J].Comparative Biochemistry and Physiology Part A:Molecular and Integrative Physiology,2013,164(4):612-621.
[39] DIONNE-WILSON L.Serotonin as a regulator of the hypothalamic-pituitary-interrenal axis in rainbow trout (Oncorhynchus mykiss)[D].Ottawa:University of Ottawa,2015.
[40] POPOVA N K.From genes to aggressive behavior:the role of serotonergic system[J].Bioessays,2006,28(5):495-503.
[41] MAXIMINO C,DA SILVA A W B,GOUVEIA JR A,et al.Pharmacological analysis of zebrafish (Danio rerio)scototaxis[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2011,35(2):624-631.
[42] CONNORS K A,VALENTI T W,LAWLESS K,et al.Similar anxiolytic effects of agonists targeting serotonin 5-HT1A or cannabinoid CB receptors on zebrafish behavior in novel environments[J].Aquatic Toxicology,2014,151:105-113.
[43] THÖRNQVIST P O,HÖGLUND E,WINBERG S.Natural selection constrains personality and brain gene expression differences in Atlantic salmon (Salmo salar)[J].Journal of Experimental Biology,2015,218(7):1077-1083.
[44] MAXIMINO C,PUTY B,BENZECRY R,et al.Role of serotonin in zebrafish (Danio rerio)anxiety:relationship with serotonin levels and effect of buspirone,WAY 100635,SB 224289,fluoxetine and para-chlorophenylalanine (pCPA)in two behavioral models[J].Neuropharmacology,2013,71:83-97.
[45] NOWICKI M,TRAN S,MURALEETHARAN A,et al.Serotonin antagonists induce anxiolytic and anxiogenic-like behavior in zebrafish in a receptor-subtype dependent manner[J].Pharmacology Biochemistry and Behavior,2014,126:170-180.
[46] NATHAN F M,OGAWA S,PARHAR I S.Kisspeptin1 modulates odorant-evoked fear response via two serotonin receptor subtypes (5-HT1A and 5-HT2)in zebrafish[J].Journal of Neurochemistry,2015,133(6):870-878.
[47] KYZAR E,COLLINS C,GREEN J,et al.Effects of the hallucinogenic drugs mescaline,phencyclidine and psilocybin on zebrafish behavior and physiology[J].Faseb Journal,2012,26(S1):1043.3.https://doi.org/10.1096/fasebj.26.1_supplement.1043.3.
[48] WHITAKER K W,NEUMEISTER H,HUFFMAN L S,et al.Serotonergic modulation of startle-escape plasticity in an African cichlid fish:a single-cell molecular and physiological analysis of a vital neural circuit[J].Journal of Neurophysiology,2011,106(1):127-137.
[49] ISHIKAWA C,SHIGA T.The postnatal 5-HT1A receptor regulates adult anxiety and depression differently via multiple molecules[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2017,78:66-74.
[50] BEULIG A,FOWLER J.Fish on prozac:effect of serotonin reuptake inhibitors on cognition in goldfish[J].Behavioral Neuroscience,2008,122(2):426.
[51] BARBA-ESCOBEDO P A,GOULD G G.Visual social preferences of lone zebrafish in a novel environment:strain and anxiolytic effects[J].Genes Brain and Behavior,2012,11(3):366-373.
[52] BEST J D,ALDERTON W K.Zebrafish:an in vivo model for the study of neurological diseases[J].Neuropsychiatric Disease and Treatment,2008,4(3):567.
[53] DE ABREU M S,MAXIMINO C,CARDOSO S C,et al.Dopamine and serotonin mediate the impact of stress on cleaner fish cooperative behavior[J].Hormones and Behavior,2020,125:104813.
[54] PIMENTEL A F N,DOS SANTOS CARVALHO T,LIMA F,et al.Conditional approach as cooperation in predator inspection:a role for serotonin?[J].Behavioural Brain Research,2019,365:164-169.
[55] P REZ-MACEIRA J J,MANCEBO M J,ALDEGUNDE M.Serotonin-induced brain glycogenolysis in rainbow trout (Oncorhynchus mykiss)[J].Journal of Experimental Biology,2012,215(17):2969-2979.
REZ-MACEIRA J J,MANCEBO M J,ALDEGUNDE M.Serotonin-induced brain glycogenolysis in rainbow trout (Oncorhynchus mykiss)[J].Journal of Experimental Biology,2012,215(17):2969-2979.
[56] SHI C,GAO X L,LIU Y,et al.Long-term monitoring of the individual self-feeding behavior of rainbow trout Oncorhynchus mykiss[J].Journal of Oceanology and Limnology,2019,37(1):344-349.
[57] MACEIRA J J P,MANCEBO M J,ALDEGUNDE M.The involvement of 5-HT-like receptors in the regulation of food intake in rainbow trout (Oncorhynchus mykiss)[J].Comparative Biochemistry and Physiology Part C:Toxicology and Pharmacology,2014,161:1-6.
[58] P REZ-MACEIRA J J,OTERO-RODI
REZ-MACEIRA J J,OTERO-RODI O C,MANCEBO M J,et al.Food intake inhibition in rainbow trout induced by activation of serotonin 5-HT2C receptors is associated with increases in POMC,CART and CRF mRNA abundance in hypothalamus[J].Journal of Comparative Physiology B,2016,186(3):313-321.
O C,MANCEBO M J,et al.Food intake inhibition in rainbow trout induced by activation of serotonin 5-HT2C receptors is associated with increases in POMC,CART and CRF mRNA abundance in hypothalamus[J].Journal of Comparative Physiology B,2016,186(3):313-321.
[59] JONNAKUTY C,GRAGNOLI C.What do we know about serotonin?[J].Journal of Cellular Physiology,2008,217(2):301-306.
[60] VELARDE E,DELGADO M J,ALONSO-G MEZ A L.Serotonin-induced contraction in isolated intestine from a teleost fish (Carassius auratus):characterization and interactions with melatonin[J].Neurogastroenterology and Motility,2010,22(12):e364-e373.
MEZ A L.Serotonin-induced contraction in isolated intestine from a teleost fish (Carassius auratus):characterization and interactions with melatonin[J].Neurogastroenterology and Motility,2010,22(12):e364-e373.
[61] EL-NACHEF W N,BRONNER M E.De novo enteric neurogenesis in post-embryonic zebrafish from schwann cell precursors rather than resident cell types[J].Development,2020,147(13):dev186619.
[62] FRITSCHE R,REID S G,THOMAS S,et al.Serotonin-mediated release of catecholamines in the rainbow trout Oncorhynchus mykiss[J].Journal of Experimental Biology,1993,178(1):191-204.
[63] KHAN N,DESCHAUX P.Role of serotonin in fish immunomodulation[J].Journal of Experimental Biology,1997,200(13):1833-1838.
[64] DUFFY-WHRITENOUR J E,ZELIKOFF J T.Relationship between serotonin and the immune system in a teleost model[J].Brain Behavior and Immunity,2008,22(2):257-264.
[65] FERRIERE F,KHAN N A,TROUTAUD D,et al.Serotonin modulation of lymphocyte proliferation via 5-HT1A receptors in rainbow trout (Oncorhynchus mykiss)[J].Developmental and Comparative Immunology,1996,20(4):273-283.
[66] MAGER E M,MEDEIROS L R,LANGE A P,et al.The toadfish serotonin 2A (5-HT2A)receptor:molecular characterization and its potential role in urea excretion[J].Comparative Biochemistry and Physiology Part A:Molecular and Integrative Physiology,2012,163(3/4):319-326.
[67] CHANDY K G,DECOURSEY T E,CAHALAN M D,et al.Electroimmunology:the physiologic role of ion channels in the immune system[J].The Journal of Immunology,1985,135(2):787S-791S.
[68] BOLANOS F J,SCHECHTER L E,MIQUEL M C,et al.Common pharmacological and physico-chemical properties of 5-HT3 binding sites in the rat cerebral cortex and NG 108-15 clonal cells[J].Biochemical Pharmacology,1990,40(7):1541-1550.
[69] MEYNIEL J P,KHAN N A,FERRI RE F,et al.Identification of lymphocyte 5-HT3 receptor subtype and its implication in fish T-cell proliferation[J].Immunology Letters,1997,55(3):151-160.
RE F,et al.Identification of lymphocyte 5-HT3 receptor subtype and its implication in fish T-cell proliferation[J].Immunology Letters,1997,55(3):151-160.
[70] HERNANDEZ-RAUDA R,ALDEGUNDE M.Changes in dopamine,norepinephrine and serotonin levels in the pituitary,telencephalon and hypothalamus during gonadal development of male Lutjanus argentiventris (Teleostei)[J].Marine Biology,2002,141(2):209-216.
[71] KHAN I A,THOMAS P.Seasonal and daily variations in the plasma gonadotropin Ⅱ response to a LHRH analog and serotonin in Atlantic croaker (Micropogonias undulatus):evidence for mediation by 5-HT2 receptors[J].Journal of Experimental Zoology,1994,269(6):531-537.
[72] WONG A,MURPHY C,CHANG J,et al.Direct actions of serotonin on gonadotropin-Ⅱ and growth hormone release from goldfish pituitary cells:interactions with gonadotropin-releasing hormone and dopamine and further evaluation of serotonin receptor specificity[J].Fish Physiology and Biochemistry,1998,19(1):23-34.
[73] TSAI C L,WANG L H,CHANG C F,et al.Effects of gonadal steroids on brain serotonergic and aromatase activity during the critical period of sexual differentiation in tilapia,Oreochromis mossambicus[J].Journal of Neuroendocrinology,2000,12(9):894-898.