茎柔鱼Dosidicus gigas广泛分布于东太平洋30°N~40°S海域,栖息在海表至1 200米水层[1]。它是世界上最大的头足类渔业资源,据FAO报告显示,2018年其产量达到89.2万t[2]。茎柔鱼位于海洋生态系统中层,具有承上启下的作用,它既是大型鱼类、哺乳动物、海鸟等的捕食对象,同时自身又捕食甲壳类、鱼类、头足类等[3-4]。与此同时,作为凶猛贪婪的机会主义者,茎柔鱼对饵料没有很高的选择性,且残食现象严重[5]。
目前,柔鱼类摄食生态研究方法主要有胃含物和稳定同位素分析法,其中胃含物分析法只能对尚未消化或者难以消化的生物个体进行鉴定,因此,往往低估了摄食营养水平,此外,该分析法只能分析近期的摄食状况,而不能反映长期的摄食信息[6]。稳定同位素分析法是近年来兴起的新技术,其在海洋生态系统研究中已得到了广泛应用[7-9]。生物组织中的碳、氮稳定同位素(δ13C、δ15N)不仅能反映捕食者的食物组成,而且能揭示其一段时间内的摄食偏好[10]。δ13C在整个食物网中变动不大,主要用来指示初级消费者的食物来源[11]。δ15N在海洋生物中主要指示生物的营养水平,较高营养水平生物拥有较高的δ15N[12]。为此,本研究中利用碳、氮稳定同位素技术分析东南太平洋厄瓜多尔和秘鲁公海茎柔鱼的摄食生态及其营养生态位,以期揭示茎柔鱼随个体生长的摄食习性转变。
1 材料与方法
1.1 材料
试验用114尾茎柔鱼(厄瓜多尔公海53尾,秘鲁公海61尾)样本由中国鱿钓生产船于2019年6—12月在东南太平洋厄瓜多尔、秘鲁公海生产时采集(图1、表1)。

图1 茎柔鱼样本采集站点
Fig.1 Sampling sites of jumbo squid Dosidicus gigas
表1 东南太平洋茎柔鱼样本信息
Tab.1 Sampling information on jumbo squid Dosidicus gigas in the southeast Pacific Ocean
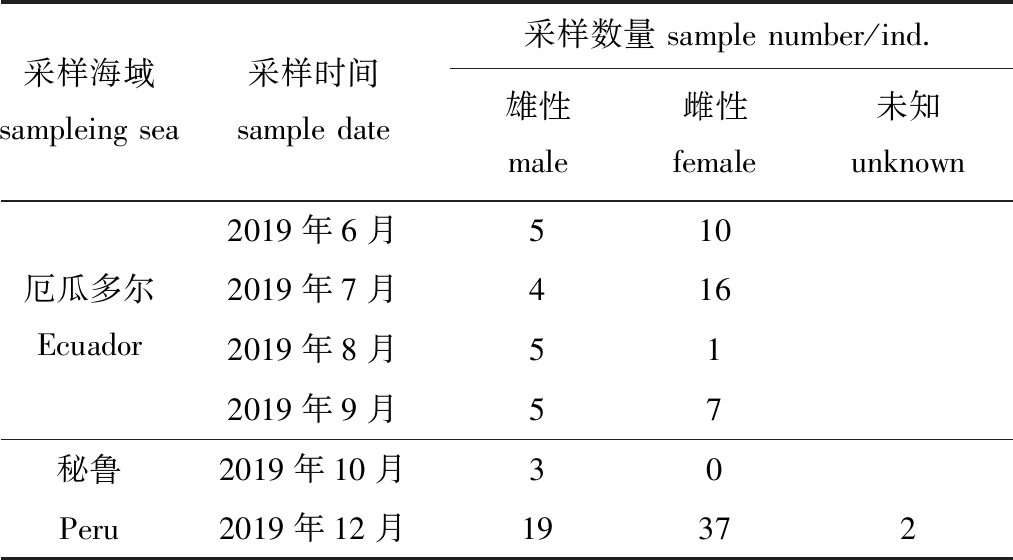
采样海域sampleing sea采样时间sample date采样数量 sample number/ind.雄性 male雌性 female未知 unknown2019年6月510厄瓜多尔Ecuador2019年7月4162019年8月512019年9月57秘鲁2019年10月30Peru2019年12月19372
1.2 方法
1.2.1 生物学测定 将采集的所有样品在上海海洋大学海洋科学学院生物学实验室解冻后进行生物学测量和观测,主要包括胴长(mm)、性腺成熟度、摄食等级等。在胴体前端剪取约2 cm×2 cm肌肉块,置于5 mL离心管中,于-20 ℃冷冻保存。
1.2.2 稳定同位素分析 肌肉去除外层表皮并用超纯水清洗,在冷冻干燥机内-55 ℃下干燥24 h后用混合型球磨仪研磨成粉末。称取1.0 mg粉末并用锡纸包被后送入稳定同位素质谱仪(ISOPRIME 100)和元素分析仪(vario ISOTOPE cube)中进行稳定同位素测定,结果以δ13C和δ15N值形式来表示,其计算公式如下:
δX=(Rsample/Rstandard-1)×1000。
(1)
其中:X为13C或15N;Rsample为13 C/12C(或15 N/14N);δ13C值以PDB值(pee dee belemnite)为标准,δ15N值以大气氮为标准。Rstandard为标准值,样品测定过程中,每10个样品放入3个实验室标准品(蛋白质 δ13C=-26.98‰,δ15N=5.96‰)校准碳、氮稳定同位素(分析精度为0.06‰),稳定同位素测定在上海海洋大学稳定同位素分析实验室进行。肌肉脂质中不包含氮,不会显著改变δ15N值,会显著改变δ13C值[13],本文中茎柔鱼脂质C∶N值范围为3.0~3.5,脂质含量较低,可以不做脱脂处理。
1.2.3 广义可加模型分析 利用广义可加模型(generalized additive model,GAM)对茎柔鱼肌肉δ13C、δ15N和胴长进行相关性分析,计算公式为
δX=s(L)+ε。
(2)
其中:δX为测定的肌肉稳定同位素值(δ13C、δ15N);s为自然立方样条平滑;L为胴长(mm)。根据F值检验评估因子影响的显著性[14-15]。
1.3 数据处理
依据Layman等[16]的方法绘制厄瓜多尔、秘鲁公海茎柔鱼δ13C、δ15N生态位宽度,并计算贝叶斯标准椭圆面积(SEAc)和重叠比例。利用t检验进行不同海域δ13C、δ15N值的显著性分析,显著性水平α=0.05。利用Kolmogorov-Smirnov检验(K-S检验)进行不同性腺等级δ13C、δ15N差异分析。利用SPSS 25.0软件进行统计分析,用R语言mgcv软件包(GAM模型)和SIBER软件包(贝叶斯标准椭圆)进行同位素绘图及分析。
2 结果与分析
2.1 稳定同位素值
厄瓜多尔、秘鲁公海茎柔鱼胴长范围分别为215~300 mm(253.8 mm±24.5 mm)、220~290 mm(254.0 mm±15.5 mm),两者不存在显著性差异(P>0.05)。厄瓜多尔公海,茎柔鱼δ13C范围为-19.81‰~-17.38‰(-18.55‰±0.56‰),δ15N范围为9.36‰~14.43‰(11.22‰±0.84‰);雄性个体δ13C为-18.71‰±0.54‰,δ15N为11.00‰±0.64‰;雌性个体δ13C为-18.36‰±0.56‰,δ15N为11.19‰±0.91‰(图2、图3)。秘鲁公海,茎柔鱼肌肉δ13C范围为-20.77‰~-17.90‰(-19.83‰±0.45‰),δ15N范围为7.65‰~13.60‰(8.97‰±1.10‰);雄性个体δ13C为-19.81‰±0.40‰,δ15N为8.82‰±0.74‰;雌性个体δ13C为-19.859‰±0.412‰,δ15N为9.053‰±1.21‰(图2、图3)。厄瓜多尔茎柔鱼肌肉的δ13C、δ15N均显著高于秘鲁公海(P<0.05)。
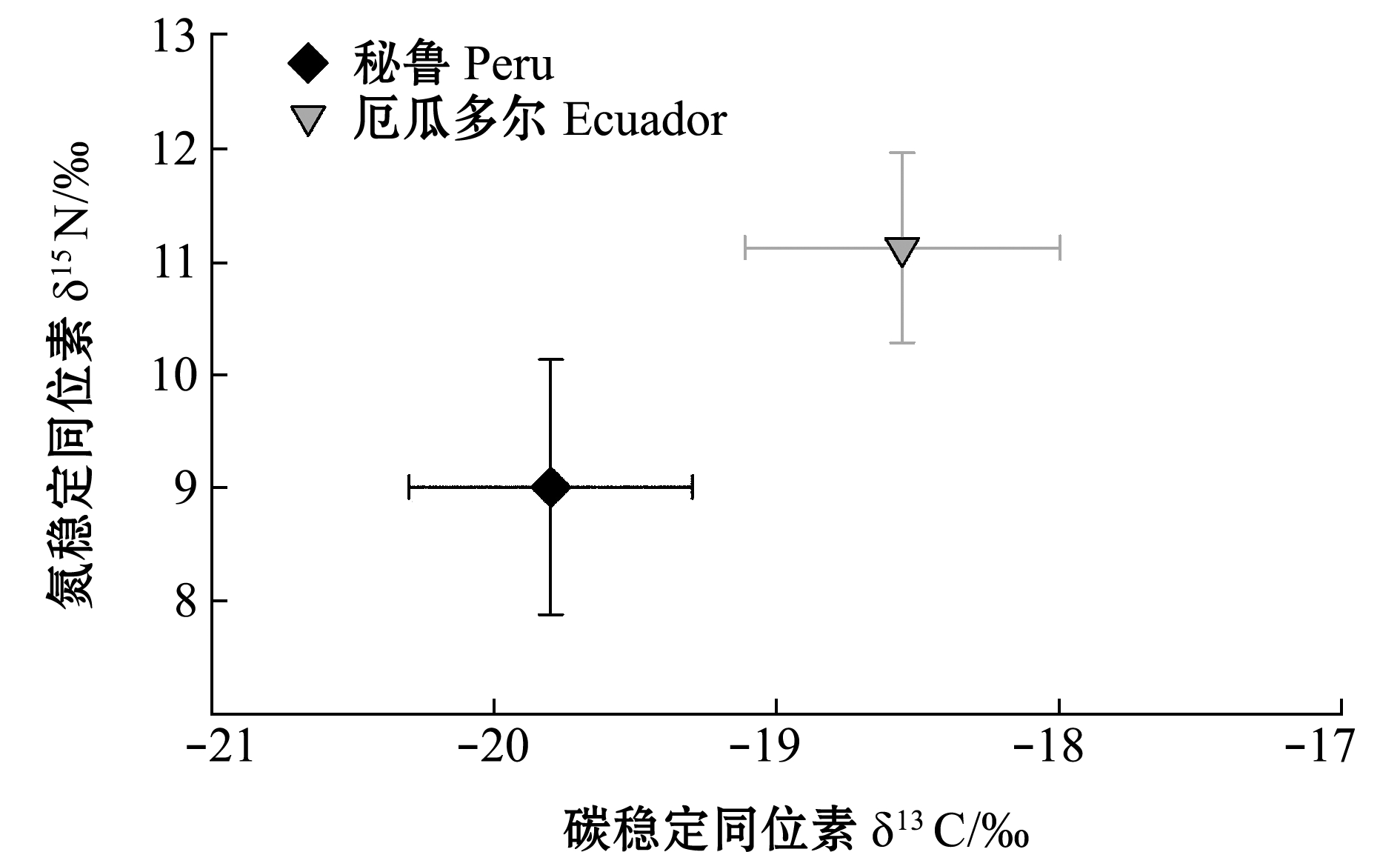
图2 东南太平洋公海茎柔鱼肌肉δ13C与δ15N的关系
Fig.2 Relationship between δ13C and δ15 N in muscle of jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
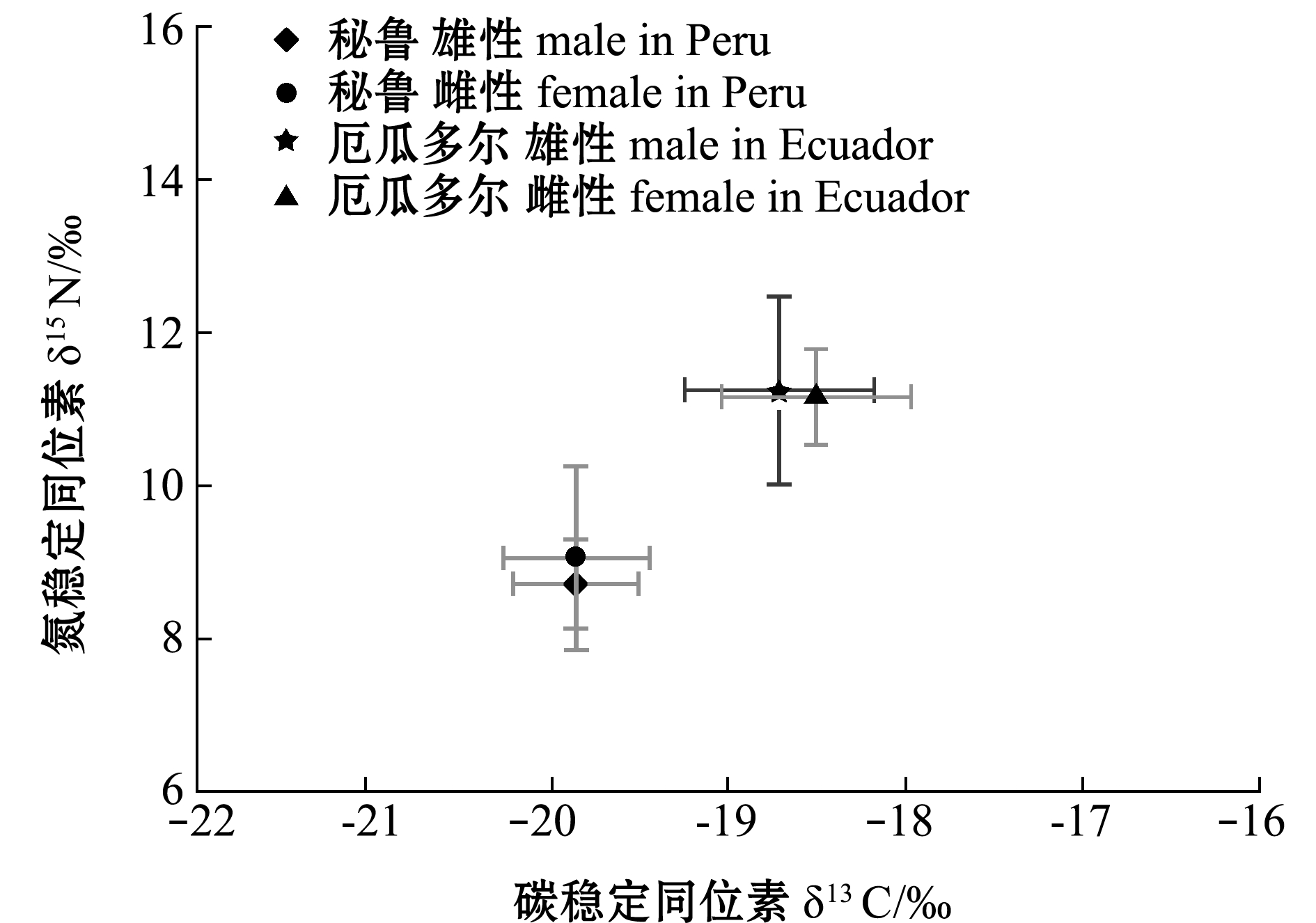
图3 东南太平洋公海茎柔鱼雌、雄个体δ13C与δ15N的关系
Fig.3 Relationship between δ13C and δ15N in female and male jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
2.2 茎柔鱼胴长对肌肉δ13C、δ15N的影响
对肌肉GAM模型分析表明:厄瓜多尔公海茎柔鱼胴长对肌肉δ13C无显著性影响(P>0.05),胴长对δ13C偏离解释率为2.11%;秘鲁公海茎柔鱼胴长对肌肉δ13C影响显著(P<0.05),δ13C在胴长220~270 mm范围内缓慢减少,胴长>270 mm时δ13C值随胴长的增长迅速升高,胴长对肌肉δ13C偏差解释率为75.80%(表2、图4)。
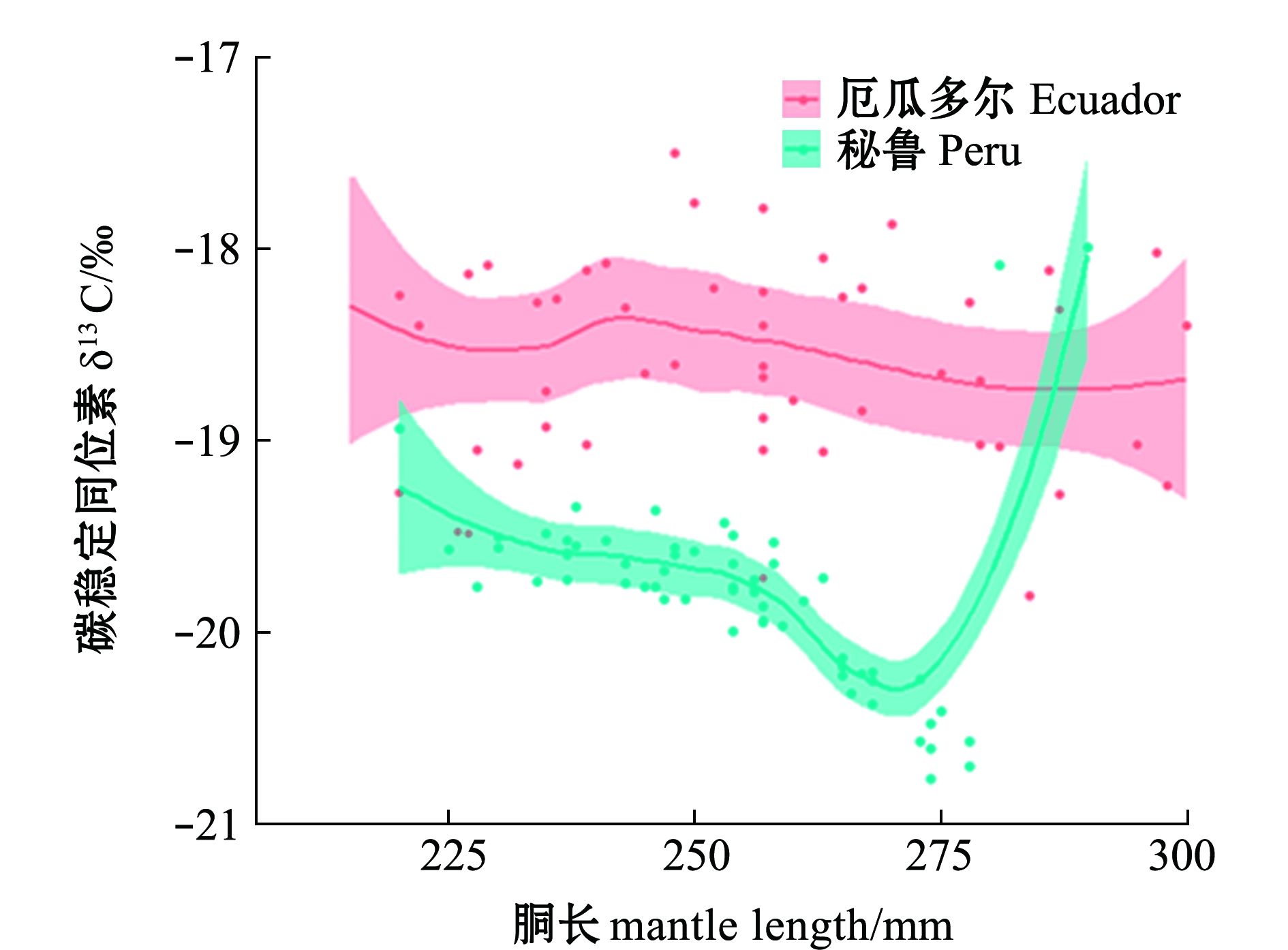
图4 东南太平洋公海茎柔鱼胴长对肌肉δ13C的影响
Fig.4 Effects of mantle length on δ13C in muscle of jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
厄瓜多尔公海茎柔鱼胴长对肌肉δ15N无显著性影响(P>0.05),胴长对肌肉δ13N偏离解释率为0.70%;秘鲁公海茎柔鱼胴长对肌肉δ15N影响显著(P<0.05),δ15N在胴长为220~230 mm时减小,胴长为230~270 mm时δ15N无显著性变化,胴长>270 mm时δ15N值随着胴长的增长而迅速升高,胴长对肌肉δ13C偏离解释率为65.10%(表2、图5)。茎柔鱼胴长在200~280 mm时,秘鲁公海茎柔鱼肌肉δ13C、δ15N低于厄瓜多尔公海;胴长>280 mm时,秘鲁公海茎柔鱼肌肉δ15N高于厄瓜多尔公海(图5)。
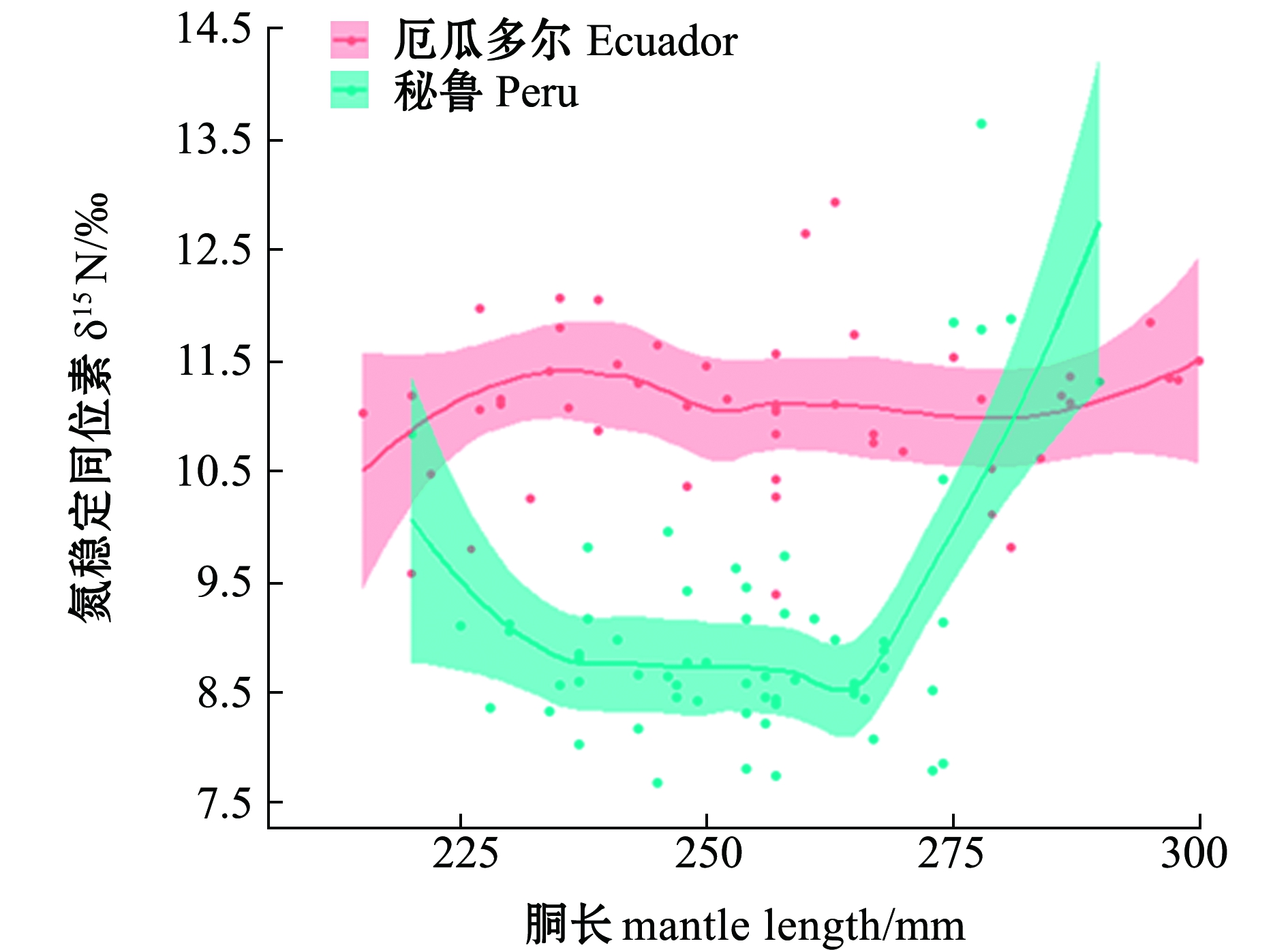
图5 东南太平洋公海茎柔鱼胴长对肌肉δ15N的影响
Fig.5 Effects of mantle length on δ15N in muscle of jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
表2 茎柔鱼胴长与肌肉δ13C、δ15N的GAM模型分析
Tab.2 GAM tests between mantle length and δ13C and δ15 N in muscle of jumbo squid Dosidicus gigas
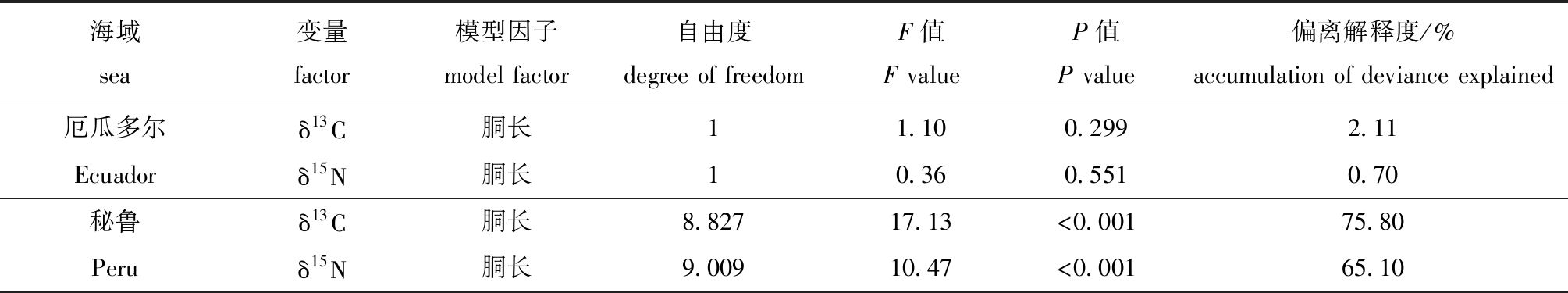
海域sea变量factor模型因子model factor自由度degree of freedomF值F valueP值P value偏离解释度/%accumulation of deviance explained厄瓜多尔δ13C胴长11.100.2992.11 Ecuadorδ15N胴长10.360.5510.70秘鲁 δ13C胴长8.82717.13<0.00175.80Peruδ15N胴长9.00910.47<0.00165.10
2.3 不同性别茎柔鱼胴长对δ13C、δ15N的影响
对性别GAM模型分析表明:在厄瓜多尔公海,雌、雄个体胴长对δ13C均无显性著影响(P>0.05),雄性个体胴长对δ15N无显著性影响(P>0.05),但雌性个体胴长对δ15N有显著性影响(P<0.05),在秘鲁公海,茎柔鱼雌、雄个体胴长对δ13C、δ15N均无显著性影响(P>0.05),茎柔鱼雌、雄个体δ13C在胴长250 mm附近达到最大值,雌、雄个体δ15N在胴长220~270 mm范围内无显著性变化(表3、图6)。
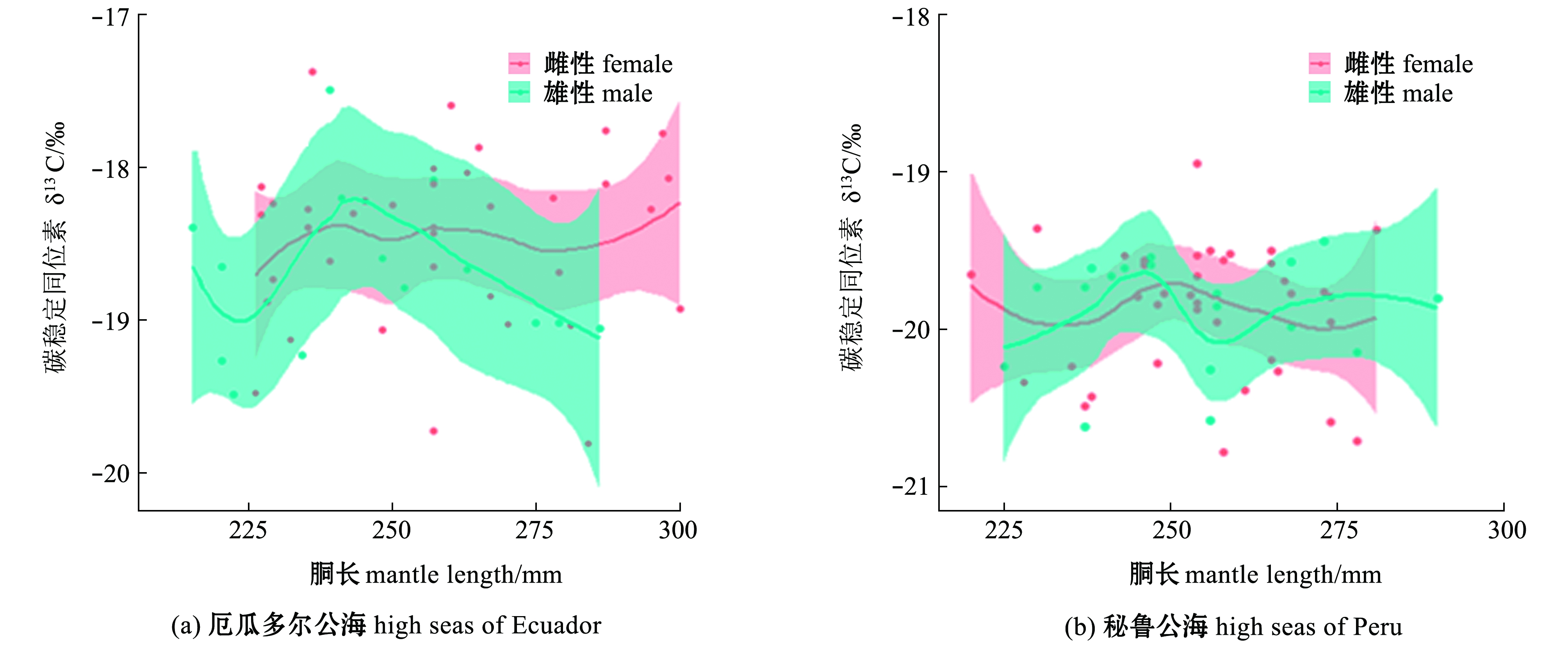
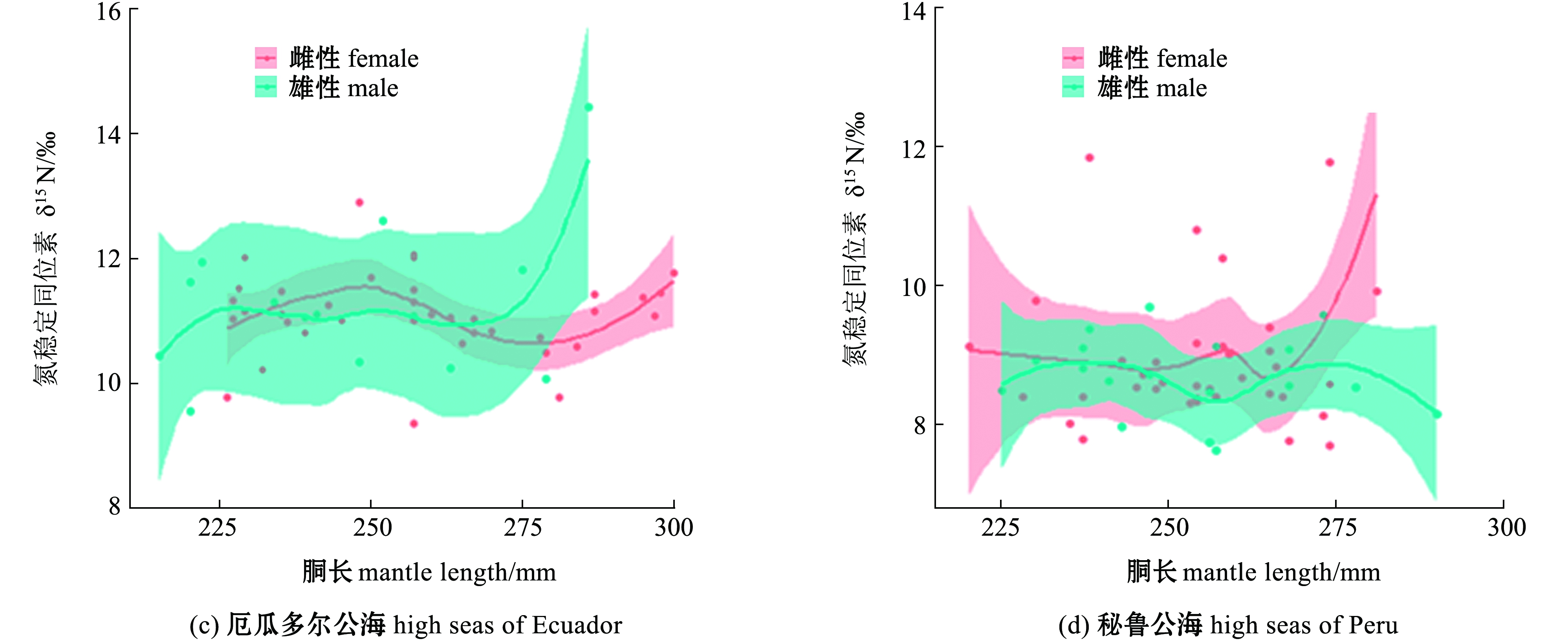
图6 东南太平洋公海茎柔鱼胴长对雌、雄个体肌肉δ13C、δ15N的影响
Fig.6 Effects of mantle length on δ13C and δ15N in muscle of female and male jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
表3 茎柔鱼雌、雄个体肌肉δ13C、δ15N与胴长的GAM模型分析
Tab.3 GAM tests of δ13C and δ15N in muscle of female and male of mantle length in jumbo squid Dosidicus gigas
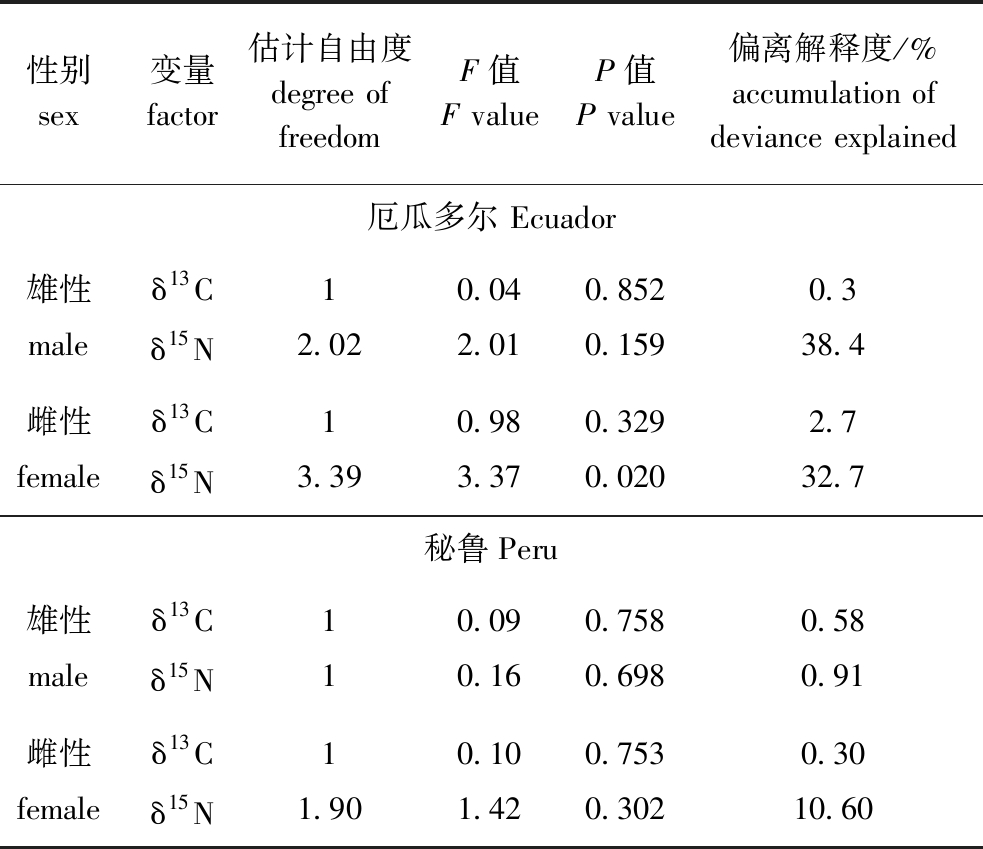
性别sex变量factor估计自由度degree of freedomF值F valueP值P value偏离解释度/%accumulation of deviance explained厄瓜多尔 Ecuador雄性maleδ13Cδ15N12.020.042.010.8520.1590.338.4雌性femaleδ13Cδ15N13.390.983.370.3290.0202.732.7秘鲁Peru雄性 maleδ13Cδ15N110.090.160.7580.6980.580.91雌性femaleδ13Cδ15N11.900.101.420.7530.3020.3010.60
2.4 营养生态位
基于茎柔鱼肌肉δ13C、δ15N绘制的贝叶斯标准椭圆显示(图7),秘鲁公海茎柔鱼生态位(SEAc=2.182‰)宽度大于厄瓜多尔公海(SEAc=1.462‰),两者重叠比例为16.12%。厄瓜多尔公海茎柔鱼雌性个体生态位(SEAc=1.672‰)宽度大于雄性个体(SEAc=0.662‰),两者重叠比例为35.73%;秘鲁公海雌性个体生态位宽度(SEAc=1.542‰)大于雄性个体(SEAc=0.632‰),两者重叠比例为34.76%;两海域雌性个体生态位均高于雄性个体(图8)。

图7 东南太平洋公海茎柔鱼营养生态位
Fig.7 Trophic niche of jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
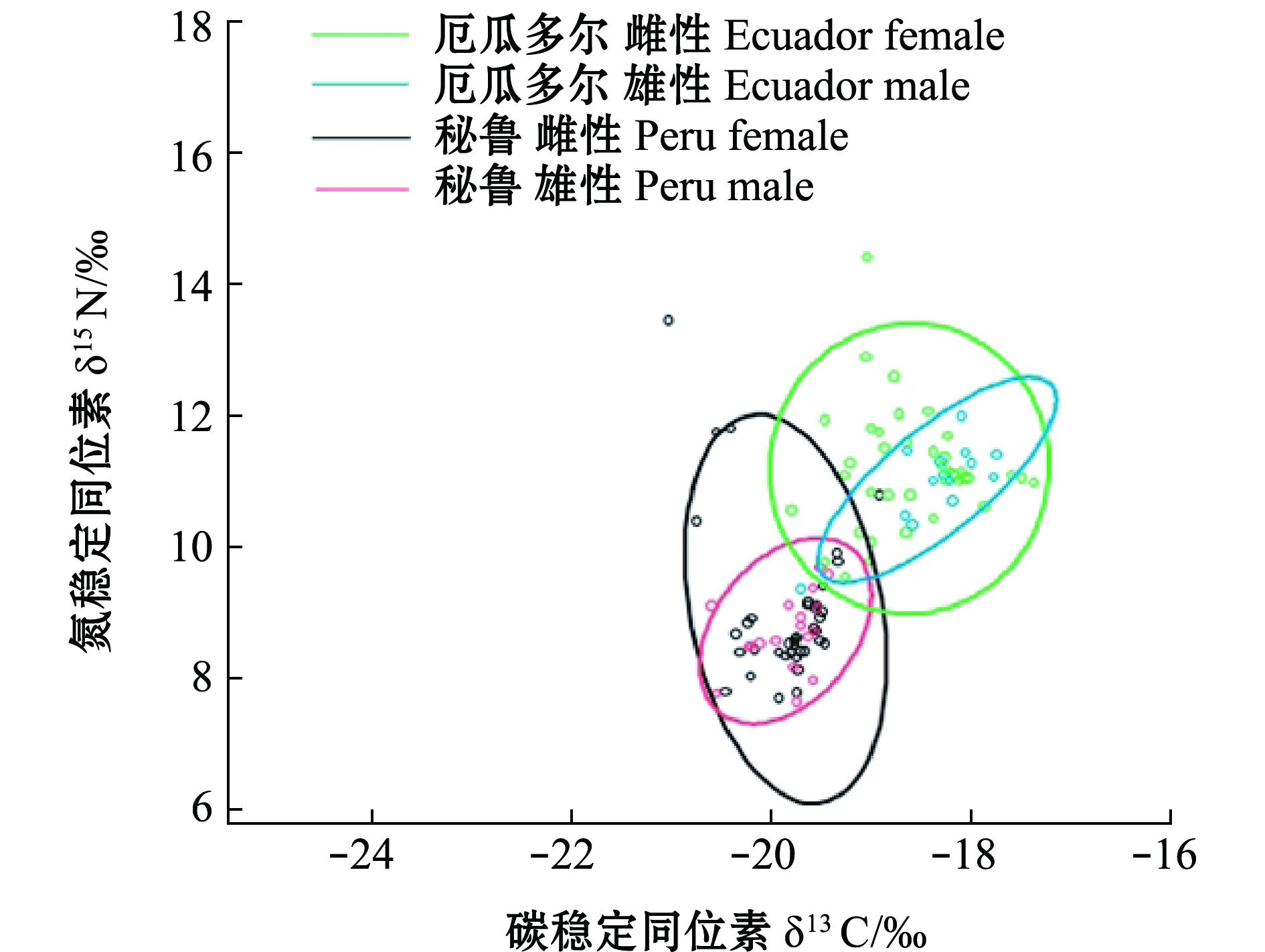
图8 东南太平洋公海茎柔鱼雌、雄个体营养生态位
Fig.8 Trophic niche of male and female jumbo squid Dosidicus gigas in the high seas of southeast Pacific Ocean
2.5 不同性腺等级间茎柔鱼δ13C、δ15N的差异
厄瓜多尔、秘鲁公海茎柔鱼性腺等级为Ⅰ~Ⅳ期,其中Ⅰ和Ⅱ期未成熟个体较多,缺少Ⅴ期成熟个体(图9)。在厄瓜多尔公海,茎柔鱼随着个体发育,δ13C在Ⅰ~Ⅲ期无显著性差异(P>0.05),δ15N在Ⅰ~Ⅲ期随着性腺等级增加显著减小(P<0.05);在秘鲁公海,茎柔鱼δ13C在性腺等级Ⅲ期最大(P<0.05),而δ15N在性腺等级Ⅱ期最大(P<0.05),此后随着性腺等级的增加,δ15N开始减小。
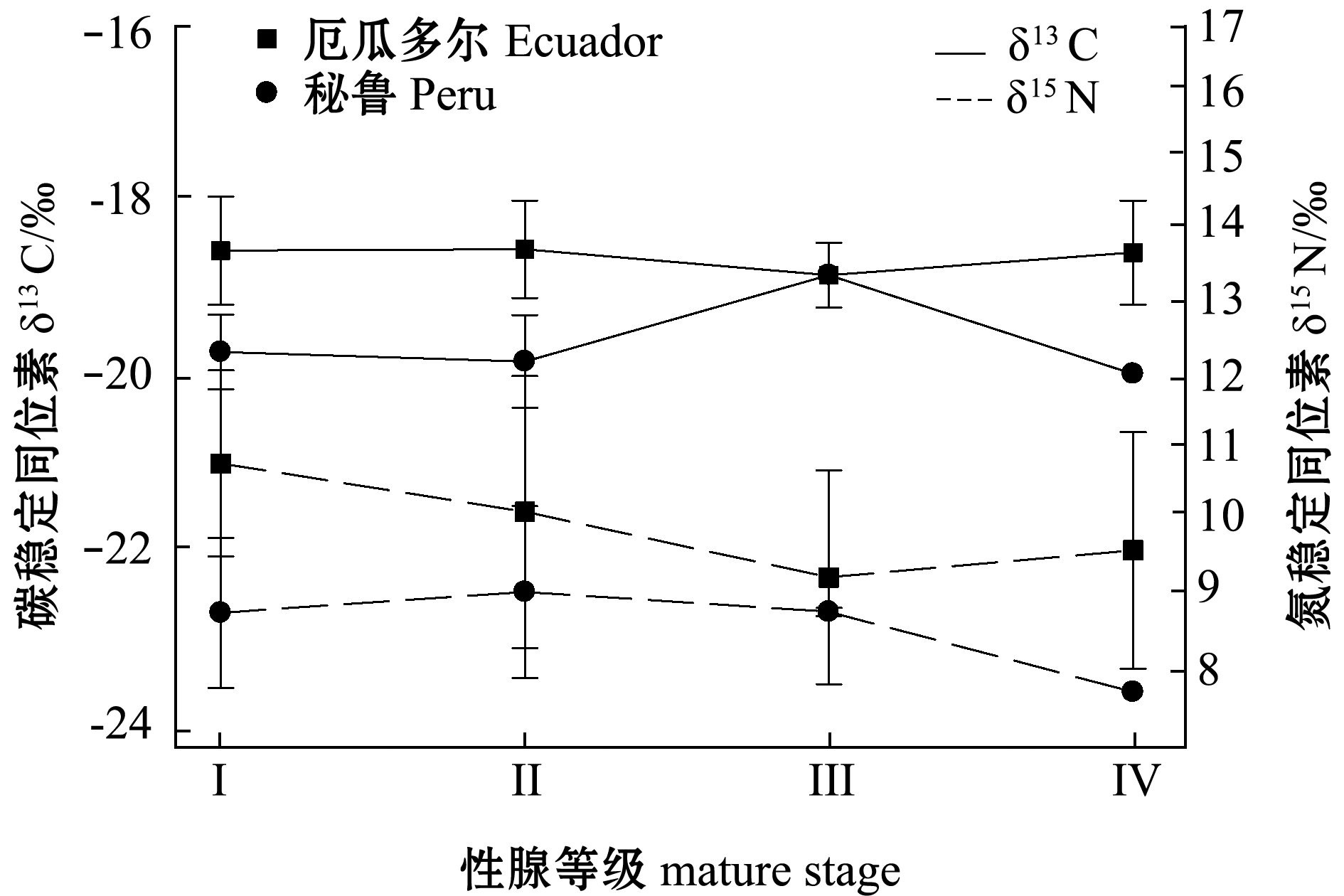
图9 茎柔鱼不同性腺等级δ13C、δ15N分布
Fig.9 δ13C and δ15N of jumbo squid Dosidicus gigas with different gonadal maturity
3 讨论
3.1 稳定同位素海域差异
生物组织中的稳定同位素值反映了基线值的空间变化和营养关系[17]。秘鲁寒流生态系统是全球海洋中重要的沿岸上升流系统之一[18],这一海域在光合作用的影响下,浮游植物优先吸收12C,富含硝酸盐的深层海水流向海表增加了浮游植物的生产力,使海域13C值显著高于非上升流海域[19-20]。东南太平洋海域分布着世界上最大和最小含氧层(OMZs)[21],OMZs 是氮![]() 通过反硝化(denitrification)和厌氧氨氧化(anammox)向大气流失的主要区域[22],氮元素释放到海洋生态系统中被生物吸收,造成生物δ15N值偏高[23]。本研究中,尽管厄瓜多尔和秘鲁公海茎柔鱼胴长无显著性差异,然而厄瓜多尔公海茎柔鱼肌肉δ13C、δ15N值显著高于秘鲁公海,这与之前文献研究结果不同,如Ruiz-Cooley等[17]对这一海域茎柔鱼肌肉稳定同位素分析发现,秘鲁公海茎柔鱼肌肉δ13C、δ15N高于厄瓜多尔公海,Liu等[24]对东南太平洋海域茎柔鱼角质颚稳定同位素发现,茎柔鱼在秘鲁公海具有较高的δ13C、δ15N值。尽管上述研究中秘鲁公海茎柔鱼大多数胴长大于厄瓜多尔公海,造成茎柔鱼肌肉δ13C、δ15N值在秘鲁公海大于厄瓜多尔公海,但在少部分相同胴长范围内,秘鲁公海δ13C、δ15N值仍大于厄瓜多尔公海。本研究中,秘鲁公海茎柔鱼样本于厄尔尼诺气候条件下采集,在此气候条件下,深层海水上涌减少,盐分等营养物质减少,群落初级生产力减弱。此外,海洋OMZs下移,茎柔鱼在更深的水层进行摄食洄游,垂直洄游距离增加[25],这些可能是造成秘鲁公海茎柔鱼肌肉δ13C、δ15N显著减小的主要原因。
通过反硝化(denitrification)和厌氧氨氧化(anammox)向大气流失的主要区域[22],氮元素释放到海洋生态系统中被生物吸收,造成生物δ15N值偏高[23]。本研究中,尽管厄瓜多尔和秘鲁公海茎柔鱼胴长无显著性差异,然而厄瓜多尔公海茎柔鱼肌肉δ13C、δ15N值显著高于秘鲁公海,这与之前文献研究结果不同,如Ruiz-Cooley等[17]对这一海域茎柔鱼肌肉稳定同位素分析发现,秘鲁公海茎柔鱼肌肉δ13C、δ15N高于厄瓜多尔公海,Liu等[24]对东南太平洋海域茎柔鱼角质颚稳定同位素发现,茎柔鱼在秘鲁公海具有较高的δ13C、δ15N值。尽管上述研究中秘鲁公海茎柔鱼大多数胴长大于厄瓜多尔公海,造成茎柔鱼肌肉δ13C、δ15N值在秘鲁公海大于厄瓜多尔公海,但在少部分相同胴长范围内,秘鲁公海δ13C、δ15N值仍大于厄瓜多尔公海。本研究中,秘鲁公海茎柔鱼样本于厄尔尼诺气候条件下采集,在此气候条件下,深层海水上涌减少,盐分等营养物质减少,群落初级生产力减弱。此外,海洋OMZs下移,茎柔鱼在更深的水层进行摄食洄游,垂直洄游距离增加[25],这些可能是造成秘鲁公海茎柔鱼肌肉δ13C、δ15N显著减小的主要原因。
3.2 茎柔鱼胴长对肌肉δ13C、δ15N的影响
海洋和生物地球化学过程的差异会导致基线生物δ13C和δ15N发生强烈的时空变化[26]。Argüelles等[27]对东太平洋厄瓜多尔沿岸茎柔鱼研究发现,δ15N存在显著的纬度梯度变化。本研究中GAM模型结果显示,随着胴长的增加,厄瓜多尔公海茎柔鱼肌肉δ13C、δ15N无显著变化,这表明厄瓜多尔公海在同位素基线和摄食作用的共同影响下,茎柔鱼肌肉δ13C、δ15N在胴长组间的差异小。本研究中秘鲁公海57条茎柔鱼样本于同一站点采集,海洋同位素基线对茎柔鱼肌肉δ13C、δ15N影响小,摄食差异是茎柔鱼不同胴长组间δ13C、δ15N差异的重要原因,秘鲁公海茎柔鱼肌肉δ13C在胴长200~270 mm范围内显著减小,表明小个体茎柔鱼在近岸水域进行摄食,随着胴长的增长,茎柔鱼具有不同的摄食和洄游方式,这与Liu等[28]的研究结果一致;当胴长>270 mm时,茎柔鱼肌肉δ13C值快速升高,表明大个体茎柔鱼向近岸海域洄游进行摄食,大个体茎柔鱼具有较为广泛的洄游和摄食活动。本研究中秘鲁公海茎柔鱼δ15N在胴长230~270 mm范围内无显著性变化,表明小个体茎柔鱼摄食结构单一,当胴长>270 mm时,茎柔鱼肌肉δ15N随着胴长的增大而显著升高,表明大个体茎柔鱼摄食营养能力迅速提高,胴长>280 mm时,秘鲁公海茎柔鱼肌肉δ15N高于厄瓜多尔公海,表明在这一阶段秘鲁公海茎柔鱼摄食更高营养级的食物。值得一提的是,在厄尔尼诺气候条件下,尽管食物资源匮乏,茎柔鱼可能通过残食快速获取食物,这与Markaida等[29]分析厄尔尼诺气候条件下茎柔鱼胃含物的结果一致。本研究中厄瓜多尔公海,茎柔鱼胴长对雌、雄个体肌肉δ13C无显著性影响,胴长>250 mm时,茎柔鱼雄性个体 δ13C开始减小,胴长>270 mm时,雄性个体δ15N大于雌性个体。茎柔鱼雄性个体的发育要早于雌性个体[30-31],随着雄性个体的发育,雄性个体开始向较深水域移动摄食高营养的食物资源。本研究中秘鲁公海茎柔鱼胴长对δ13C、δ15N无显著性影响,δ13C在雌、雄个体间差异不大,表明厄尔尼诺气候条件下会从摄食空间、时间及食物资源方面限制茎柔鱼摄食和洄游空间[32-33],胴长>270 mm时,雌性个体δ15N开始增加,雌性个体摄食水平提高。
3.3 生态位海域差异
Fang等[34]对北太平洋海域柔鱼秋生群体和冬春群体角质颚δ13C、δ15N研究发现,两个群体存在生态位差异,这种差异可能是不同地理种群的不同生长速率、洄游路线和摄食差异所导致。Gong等[35]对东南太平洋茎柔鱼肌肉稳定同位素研究发现,茎柔鱼在秘鲁公海具有较高的生态位。本研究发现,厄瓜多尔公海茎柔鱼肌肉δ13C、δ15N值显著高于秘鲁公海,厄瓜多尔公海茎柔鱼具有较低的生态位,表明其受到同位素基线和摄食作用的共同影响,δ13C、δ15N在胴长间差异小,表明茎柔鱼具有较低的生态位。秘鲁公海茎柔鱼采集的样本中,海洋同位素基线差异小,因此,摄食差异可能是这一海域茎柔鱼生态位差异的重要原因。本研究中,当胴长>270 mm,秘鲁公海茎柔鱼δ13C、δ15N显著升高,表明厄尔尼诺气候条件下,茎柔鱼可能通过残食来提高摄食营养,茎柔鱼肌肉δ13C、δ15N在胴长组间差异大,表明茎柔鱼具有较高的生态位。雌性和雄性生物位于不同栖息环境可以减少种内竞争[36-37],本研究中发现,厄瓜多尔、秘鲁公海,茎柔鱼雌性个体生态位均高于雄性个体。Fang等[38]发现,北太平洋柔鱼雌性个体比雄性个体摄食器官角质颚尺寸更大,雌性个体摄食能力较强。茎柔鱼雌性个体在生长过程中能量消耗大,对食物资源和能量需求较高[39]。随着生长过程中的食物需求和摄食能力的提高,茎柔鱼雌性个体具有较高的生态位。
3.4 不同性腺等级间茎柔鱼δ13C、δ15N的差异
短生命周期的头足类生长速率快,能量代谢率高,通过摄食用于个体生长发育和繁殖[31,40]。本研究中厄瓜多尔公海,茎柔鱼随着个体发育,在性腺等级Ⅰ~Ⅲ期时,茎柔鱼δ13C值无明显变化,茎柔鱼栖息环境无显著变化,但在性腺等级Ⅲ~Ⅳ期时茎柔鱼开始向高生产力海域进行洄游摄食,这与Liu等[41]的研究结果一致。方舟等[42]对柔鱼摄食器官角质颚研究发现,在不同性腺等级个体间其角质颚形态存在显著差异。这说明随着摄食器官的不断完善,茎柔鱼摄食能力和摄食强度开始提高,茎柔鱼开始摄食较大和较高营养的饵料生物。本研究中,茎柔鱼个体在性腺等级Ⅰ~Ⅳ期,茎柔鱼δ15N无显著变化,可能是受到海域同位素基线作用的影响。而秘鲁公海茎柔鱼δ13C值在性腺等级Ⅰ~Ⅲ期随个体发育程度增加而增加,这表明在厄尔尼诺气候条件下茎柔鱼开始向近岸等较高生产力水域进行洄游,这与Hu等[43]对厄尔尼诺气候条件下茎柔鱼角质颚δ13C的研究结果一致。茎柔鱼δ15N在Ⅲ期最大,随着个体性腺成熟度的增加,δ15N开始减小,表明茎柔鱼从较高的营养水域向低营养水域进行洄游,这与Gong等[40]的研究结果一致。东南太平洋公海,茎柔鱼向近岸水域进行洄游摄食,然后洄游到产卵场的季节性迁徙[27]。在这一过程中,海洋基线δ15N开始减小,在厄尔尼诺气候条件下,由于食物资源的匮乏,茎柔鱼δ15N受摄食因素的影响开始减少。
4 结论
1)本研究中,厄瓜多尔公海茎柔鱼胴长对肌肉 δ13C、δ15N无显著性影响(P>0.05),而秘鲁公海茎柔鱼胴长则对肌肉δ13C、δ15N影响显著(P<0.05)。
2)厄瓜多尔和秘鲁公海茎柔鱼生态位存在差异,秘鲁公海生态位宽度大于厄瓜多尔公海,雌性个体茎柔鱼在两个海域均有较高的生态位。
3)厄瓜多尔公海茎柔鱼δ13C、δ15N受同位素基线和摄食作用共同影响,秘鲁公海茎柔鱼δ13C、δ15N主要受摄食作用影响。
4)一般地,脂质的存在会影响肌肉中的δ13C值,而对δ15N值无影响。本研究中茎柔鱼脂质C∶N值范围为3.0~3.5,脂质含量较低,可以不做脱脂处理,但是也有研究指出,茎柔鱼肌肉脱脂处理后会对δ13C、δ15N产生一定影响[44],因此,今后进行头足类肌肉碳、氮稳定同位素分析试验时,应尽可能进行脱脂处理。
[1] JEREB P,ROPER C F E.Cephalopods of the world.an annotated and illustrated catalogue of cephalopod species known to date.volume 2.myopsid and oegopsid squids:FAO species catalogue for fishery purposes[M].Rome,Italy:FAO,2010.
[2] FAO.The state of world fisheries and aquaculture 2020[R].Rome:FAO,2020.
[3] NIGMATULLINC M,NESIS K N,ARKHIPKIN A I.A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda:Ommastrephidae)[J].Fisheries Research,2001,54(1):9-19.
[4] KEAR A J,BRIGGS D E G,DONOVAN D T.Decay and fossilization of non-mineralized tissue in coleoid cephalopods[J].Palaeontology,1995,38:105-132.
[5] DAWE E G.Length-weight relationships for short-finned squid in Newfoundland and the effect of diet on condition and growth[J].Transactions of the American Fisheries Society,1988,117(6):591-599.
[6] JACKSONG D,BUSTAMANTE P,CHEREL Y,et al.Applying new tools to cephalopod trophic dynamics and ecology:perspectives from the Southern Ocean Cephalopod Workshop,February 2-3,2006[J].Reviews in Fish Biology and Fisheries,2007,17(2/3):79-99.
[7] ROUNICKJ S,WINTERBOURN M J.Stable carbon isotopes and carbon flow in ecosystems[J].BioScience,1986,36(3):171-177.
[8] POSTD M.Using stable isotopes to estimate trophic position:models,methods,and assumptions[J].Ecology,2002,83(3):703-718.
[9] 李忠义,金显仕,庄志猛,等.稳定同位素技术在水域生态系统研究中的应用[J].生态学报,2005,25(11):3052-3060.
LI Z Y,JIN X S,ZHUANG Z M,et al.Application of stable isotope technology in the study of aquatic ecosystems[J].Acta Ecologica Sinica,2005,25(11):3052-3060.(in Chinese)
[10] HOBSONK A,WELCH H E.Cannibalism and trophic structure in a high Arctic lake:insights from stable-isotope analysis[J].Canadian Journal of Fisheries and Aquatic Sciences,1995,52(6):1195-1201.
[11] HOBSONK A,PIATT J F,PITOCCHELLI J.Using stable isotopes to determine seabird trophic relationships[J].Journal of Animal Ecology,1994,63(4):786-798.
[12] WAN Y,HU J Y,AN LH,et al.Determination of trophic relationships within a Bohai Bay food web using stable δ15N and δ13C analysis[J].Chinese Science Bulletin,2005,50(10):1021-1025.
[13] WOODS N.Generalized additive models:an introduction with R[M].2nd.Floria:Chemical Rubber Company Press,2017.
[14] KILJUNEN M,GREY J,SINISALO T,et al.A revised model for lipid-normalizing δ13C values from aquatic organisms,with implications for isotope mixing models[J].Journal of Applied Ecology,2006,43(6):1213-1222.
[15] MAUNDERM N,PUNT A E.Standardizing catch and effort data:a review of recent approaches[J].Fisheries Research,2004,70(2/3):141-159.
[16] LAYMANC A,ARRINGTON D A,MONTA A C G,et al.Can stable isotope ratios provide for community-wide measures of trophic structure?[J].Ecology,2007,88(1):42-48.
A C G,et al.Can stable isotope ratios provide for community-wide measures of trophic structure?[J].Ecology,2007,88(1):42-48.
[17] RUIZ-COOLEY R I,GERRODETTE T.Tracking large-scale latitudinal patterns of δ13C and δ15N along the E Pacific using epi-mesopelagic squid as indicators[J].Ecosphere,2012,3(7):1-17.
[18] CHAVEZ F P,BERTRAND A,GUEVARA-CARRASCO R,et al.The Northern Humboldt Current System:brief history,present status and a view towards the future[J].Progress in Oceanography,2008,79(2/3/4):95-105.
[19] RAUG H,CHAVEZ F P,FRIEDERICH G E.Plankton 13C/12C variations in Monterey Bay,California:evidence of non-diffusive inorganic carbon uptake by phytoplankton in an upwelling environment[J].Deep Sea Research Part I:Oceanographic Research Papers,2001,48(1):79-94.
[20] ECHEVIN V,AUMONT O,LEDESMA J,et al.The seasonal cycle of surface chlorophyll in the Peruvian upwelling system:a modelling study[J].Progress in Oceanography,2008,79(2/3/4):167-176.
[21] KEELINGR F,KÖRTZINGER A,GRUBER N,et al.Ocean deoxygenation in a warming world[J].Annual Review of Marine Science,2010,2:199-229.
[22] STURDIVANTS K,D AZ R J,CUTTER G R,et al.Bioturbation in a declining oxygen environment,in situ observations from Wormcam[J].PLoS One,2012,7(4):e34539.
AZ R J,CUTTER G R,et al.Bioturbation in a declining oxygen environment,in situ observations from Wormcam[J].PLoS One,2012,7(4):e34539.
[23] VOSS M,DIPPNER J W,MONTOYA J P.Nitrogen isotope patterns in the oxygen-deficient waters of the Eastern Tropical North Pacific Ocean[J].Deep Sea Research Part I:Oceanographic Research Papers,2001,48(8):1905-1921.
[24] LIU B L,LI J Y,CHEN X J,et al.Inter-and intra-regional patterns of stable isotopes in Dosidicus gigas beak:biological,geographical and environmental effects[J].Marine and Freshwater Research,2018,69(3):464-472.
[25] ZEIDBERG L,HAMNER W.Distribution of squid paralarvae,Loligo opalescens (Cephalopoda:Myopsida),in the Southern California Bight in the three years following the 1997-1998 El Ni o[J].Marine Biology,2002,141(1):111-122.
o[J].Marine Biology,2002,141(1):111-122.
[26] RAUG H,TAKAHASHI T,MARAIS D J D.Latitudinal variations in plankton δ13C:implications for CO2 and productivity in past oceans[J].Nature,1989,341(6242):516-518.
[27] ARGÜELLES J,LORRAIN A,CHEREL Y,et al.Tracking habitat and resource use for the jumbo squid Dosidicus gigas:a stable isotope analysis in the Northern Humboldt Current System[J].Marine Biology,2012,159(9):2105-2116.
[28] LIU B L,JIN Y,CHEN X J,et al.High individual variability in beak stable isotopes of jumbo squid off Peruvian exclusive economic zone (EEZ)waters in the analysis of migratory and foraging ecology[J].Journal of Ocean University of China,2019,18(1):232-238.
[29] MARKAIDA U.Food and feeding of jumbo squid Dosidicus gigas in the Gulf of California and adjacent waters after the 1997-98 El Ni o event[J].Fisheries Research,2006,79(1/2):16-27.
o event[J].Fisheries Research,2006,79(1/2):16-27.
[30] TAFUR R,KEYL F,ARGÜELLES J.Reproductive biology of jumbo squid Dosidicus gigas in relation to environmental variability of the northern Humboldt Current System[J].Marine Ecology Progress Series,2010,400:127-141.
[31] 韩飞,陈新军,林东明.东太平洋赤道海域茎柔鱼组织能量积累及其与海表面环境因子的关系[J].中国水产科学,2020,27(4):427-437.
HAN F,CHEN X J,LIN D M.Tissue energy accumulation of Dosidicus gigas in the equatorial waters of the East Pacific Ocean and its relationship with environmental factors on the sea surface[J].Chinese Journal of Fishery Sciences,2020,27(4):427-437.(in Chinese)
[32] LIY K,GONG Y,ZHANG Y Y,et al.Inter-annual variability in trophic patterns of jumbo squid (Dosidicus gigas)off the exclusive economic zone of Peru,implications from stable isotope values in gladius[J].Fisheries Research,2017,187:22-30.
[33] 汪惠琼,陈洁南,李云凯,等.厄尔尼诺对柔鱼亚科近缘种茎柔鱼与鸢乌贼营养生态位的影响[J].海洋渔业,2020,42(5):524-532.
WANG H Q,CHEN J N,LI Y K,et al.The effect of El Ni o on the nutritional niche of the subfamily Dosidicus gigas and Sthenoteuthis oualaniensis[J].Marine Fisheries,2020,42(5):524-532.(in Chinese)
o on the nutritional niche of the subfamily Dosidicus gigas and Sthenoteuthis oualaniensis[J].Marine Fisheries,2020,42(5):524-532.(in Chinese)
[34] FANG Z,THOMPSON K,JIN Y,et al.Preliminary analysis of beak stable isotopes (δ13C and δ15N)stock variation of neon flying squid,Ommastrephes bartramii,in the North Pacific Ocean[J].Fisheries Research,2016,177:153-163.
[35] GONG Y,LI Y K,CHEN X J,et al.Potential use of stable isotope and fatty acid analyses for traceability of geographic origins of jumbo squid (Dosidicus gigas)[J].Rapid Communications in Mass Spectrometry,2018,32(7):583-589.
[36] CAMPHUYSEN K.Differential foraging strategies and offshore habitat preferences of seabirds feeding on sand eels in the North Sea[J].Alauda,2005,73(3):220-221.
[37] DAYAN T,SIMBERLOFF D.Ecological and community-wide character displacement:the next generation[J].Ecology Letters,2005,8(8):875-894.
[38] FANG Z,CHEN X J,SU H,et al.Evaluation of stock variation and sexual dimorphism of beak shape of neon flying squid,Ommastrephes bartramii,based on geometric morphometrics[J].Hydrobiologia,2007,784(1):367-380.
[39] O’DOR R.Cephalopod life cycles.Volume 2:Comparative reviews.P R Boyle[J].The Quarterly Review of Biology,1988,63(2):227-228.
[40] GONG Y,RUIZ-COOLEY R I,HUNSICKER M E,et al.Sexual dimorphism in feeding apparatus and niche partitioning in juvenile jumbo squid Dosidicus gigas[J].Marine Ecology Progress Series,2018,607:99-112.
[41] LIU B L,XU W,CHEN X J,et al.Ontogenetic shifts in trophic geography of jumbo squid,Dosidicus gigas,inferred from stable isotopes in eye lens[J].Fisheries Research,2020,226:105507.
[42] 方舟,陈新军,陆化杰,等.北太平洋两个柔鱼群体角质颚形态及生长特征[J].生态学报,2014,34(19):5405-5415.
FANG Z,CHEN X J,LU H J,et al.The horny jaw morphology and growth characteristics of two Ommastrephes bartramii populations in the North Pacific[J].Acta Ecologica Sinica,2014,34(19):5405-5415.(in Chinese)
[43] HU G Y,BOENISH R,GAO C X,et al.Spatio-temporal variability in trophic ecology of jumbo squid (Dosidicus gigas)in the southeastern Pacific:insights from isotopic signatures in beaks[J].Fisheries Research,2019,212:56-62.
[44] 刘娜,刘必林.脂类去除对赤道外海茎柔鱼软组织稳定同位素的影响[J].大连海洋大学学报,2021,36(1):155-160.
LIU N,LIU B L.The effect of lipid removal on stable isotopes of soft tissue of Dosidicus gigas off the equator[J].Journal of Dalian Ocean University,2021,36(1):155-160.(in Chinese)
 o climate conditions.
o climate conditions.