副乳房链球菌Streptococcus parauberis最初被认为是乳房链球菌S.uberisⅡ型[1],直到1990年Williams等[2]通过反转录酶序列分析,发现二者在系统发育上不同,并通过DNA杂交验证了二者的差异,由此命名。副乳房链球菌作为鱼类链球菌病原是在1993年西班牙养殖大菱鲆Scophthalmus maximus暴发链球菌感染之后[3],而此前一直认为该菌是引起牛乳房炎的主要病原之一[4]。目前,除牛、猪等[5-6]畜禽外,副乳房链球菌在水生动物中的主要感染对象有牙鲆Paralichthys olivaceus、虹鳟Oncorhynchus mykiss和星斑川鲽Platichthys stellatus等[7-9],该菌是引起日韩养殖牙鲆链球菌病的主要病原,曾造成严重的经济损失[7,10]。中国鱼源副乳房链球菌感染鲜有报道,笔者于2019年曾在山东省大菱鲆主产区分离到自然感染致病的副乳房链球菌菌株。结合项目组的研究及国内外有关副乳房链球菌的研究概况,本研究中主要从副乳房链球菌的病原学、流行病学与临床症状、致病机制、检测方法、耐药性、疾病防治等方面进行综述,并对未来深入开展的研究内容进行了展望,旨在为养殖生产中鱼源副乳房链球菌病的防控提供科学参考。
1 副乳房链球菌的病原学
1.1 病原特征
1)链球菌病病原及分类。链球菌属革兰氏阳性球菌,是一类可引起世界范围海淡水、野生或养殖鱼类发生类似败血病症的病原菌[11]。1958年报道了首例日本养殖虹鳟鱼感染链球菌[12],此后众多鱼类感染链球菌案例相继被报道,链球菌病已成为威胁鱼类产业并造成严重损失的重要疾病。研究表明,链球菌病可由链球菌属Streptococcus、乳球菌属Lactococci和漫游球菌属Vagococci等革兰氏阳性菌感染引起[13]。鱼类链球菌病可分为温水性和冷水性两大类,其中,鲑漫游球菌Vagococcus salmoninarum、鱼乳球菌Lactococcus piscium、格氏乳球菌L.garvieae、无乳链球菌S.agalactiac、海豚链球菌S.iniae和副乳房链球菌S.parauberis等至少6种病原菌均可引起温水性链球菌病[8]。温水性链球菌病可表现出相似的症状和临床特征,仅通过表观症状无法准确区分病原,且温水性链球菌是潜在的人畜共患病原菌,目前尚未有研究证实人源感染的菌株源于畜禽,故研究温水性链球菌对链球菌病本身及人畜共患病的防治意义重大。
2)副乳房链球菌的形态及生化特征。副乳房链球菌隶属于芽孢杆菌纲Bacilli乳杆菌目Lactobacillales链球菌科Streptococcaceae链球菌属Streptococcus,是一类兼性厌氧的革兰氏阳性菌,呈球形,直径为0.2~0.8 μm,具荚膜,无鞭毛,镜检呈短链状排列;在BHI平板中菌落形态为圆形、乳白色,菌落光滑、湿润、边缘清晰,直径约为1.2 mm(图1A、图2);该菌能在含4% NaCl培养基中生长,在含6.5% NaCl培养基或pH 9.6环境下不能生长,能利用果糖、半乳糖、麦芽糖、葡萄糖、核糖、蔗糖等产酸,不能利用肌醇、鼠李糖、山梨糖等,可水解七叶灵,VP试验呈阳性[2]。因其与乳房链球菌的相似性,拉丁名以para-为前缀,加上乳房链球菌的种名uberis,定名为副乳房链球菌S.parauberis。Williams等[2]最初对其血平板溶血特性的描述为弱的α溶血或不溶血,但后续关于副乳房链球菌溶血特性的报道多为α溶血[8-9,13-14],笔者分离到的山东地区鱼源菌株表现为不溶血[15](图1B)。Nho等[7]从具有典型链球菌症状患病牙鲆中分离到的副乳房链球菌菌株为α溶血,但回归感染后再次分离的菌株则不溶血。有研究表明,海豚链球菌中的溶血特性与基础培养基及血平板中添加的血液类型有关[16]。海豚链球菌在添加5%牛血或人血的平板中呈α溶血,而在添加羊血的平板中则呈β溶血;在3%马血心浸液平板中呈β溶血,而在添加同种同等比例血液的Todd-Hewitt平板中则呈α溶血[17]。
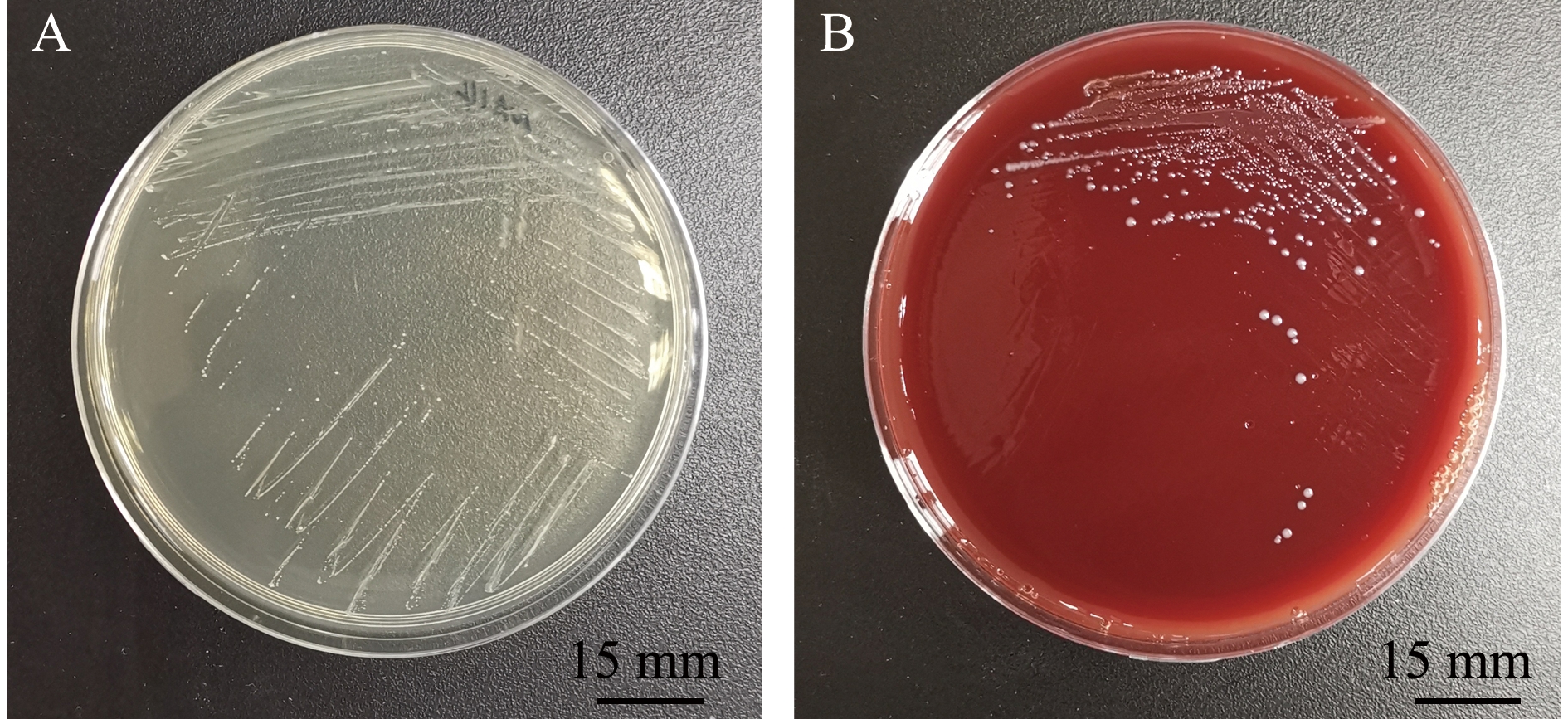
A—BHI培养基中菌落形态;B—5%羊血培养基中菌落形态。
A—colony morphology cultured with BHI culture medium; B—colony morphology cultured with 5% sheep blood plate.
图1 副乳房链球菌在培养基中的形态[15]
Fig.1 Colony morphology of Streptococcus parauberis in culture medium[15]
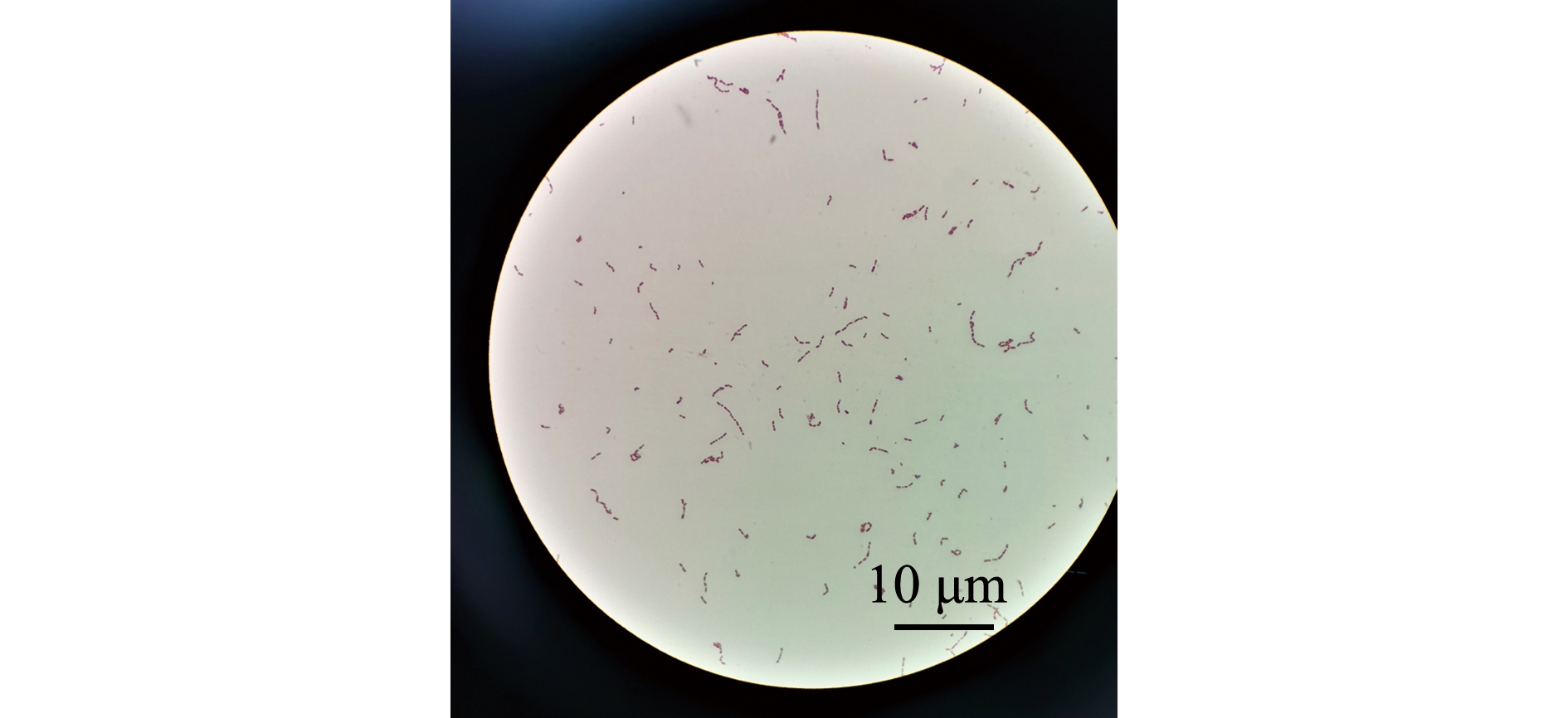
图2 副乳房链球菌的革兰氏染色形态[15]
Fig.2 Micro-morphology of Streptococcus parauberis staining with Gram[15]
1.2 分子血清型
1)血清型分型。有学者基于荚膜多糖结构和糖苷键将日本牙鲆源副乳房链球菌血清型分为Ⅰ型、Ⅱ型和未分型(non-typed, NT)3种类型[18-20],未分型是指与血清型Ⅰ或Ⅱ抗体均发生凝集反应的血清型,其中,血清型Ⅰ又可分为Ⅰa、Ⅰb、Ⅰc 3种亚型[21]。血清型Ⅰ和Ⅱ可通过Tu等[22]基于多糖聚合酶(polysaccharide polymerase gen)基因WZY设计的引物有效鉴别,其中,Ⅰa型可扩增获得213 bp产物,Ⅰb/Ⅰc型可扩增出303 bp产物,Ⅱ型可扩增出413 bp产物。然而,Yolanda等[20]用基质辅助激光解吸附电离飞行时间质谱法(matrix-assisted laser desorption Ionization-time-of-flight mass spectrometry, MALDI-TOF-MS)对不同来源副乳房链球菌血清型研究时,检测到大菱鲆源的副乳房链球菌存在不同于牙鲆源和牛源菌株的特异性质量峰,结合重复序列PCR(repetitive sequence-based PCR, Rep-PCR)、特异性蛋白生物标记物等结果,认为除了上述3种分型外,大菱鲆源菌株属于新的血清型Ⅲ,并且可通过扩增荚膜多糖基因(130 bp)与其他血清型加以辨别[23]。
2)血清型毒力。不同血清型的副乳房链球菌毒力表现不同。在同一条件下,仅血清型Ⅲ及Ⅰ菌株能感染大菱鲆发病,其中,血清型Ⅲ菌株(大菱鲆来源)对大菱鲆的致病力最强,半致死浓度为103 CFU/mL;牙鲆来源的Ⅰa、Ⅰc血清亚型分离株对大菱鲆的半致死浓度约为108 CFU/mL,牙鲆来源的Ⅰb血清亚型分离株对大菱鲆的半致死浓度超过1010 CFU/mL[20]。韩国牙鲆源分离株血清型Ⅰ毒力较强,平均凝集滴度、累计死亡率均高于Ⅱ型,对牙鲆的半致死浓度分别为107、108 CFU/mL[24]。此外,用105 CFU/mL条纹鲈源副乳房链球菌感染斑马鱼Danio rerio,不能导致其致病,剖解其脾脏组织,并不能分离到病原菌[14],提示不同来源的分离株可能存在一定的宿主特异性。
2 副乳房链球菌流行病学与临床症状
2.1 流行病学
已有报道显示,副乳房链球菌可以感染10余种鱼类致病(表1)。近年来,副乳房链球菌病在西班牙[3]、日本[9]、韩国[4]、美国、以色列、南非等多个国家和地区的鱼类养殖场中暴发流行[8],此外,也有野生鱼类检出该菌的报道[14]。在牙鲆的细菌性病害中,副乳房链球菌是引起链球菌病并导致牙鲆急性死亡的最主要病原[25],死亡率超过70%[26]。水温是链球菌病暴发的最主要诱因[27],Won等[28]报道,牙鲆26 ℃时注射副乳房链球菌的死亡率远高于22 ℃,23~24 ℃水温下副乳房链球菌感染牙鲆的半致死浓度为4.5×103 CFU/mL[29],而Hwang[30]报道的21 ℃时副乳房链球菌不同株感染牙鲆的半致死浓度为107~109 CFU/mL,低于20 ℃时的预试验中并未导致牙鲆致病[31]。尽管不同来源菌株的半致死浓度存在差异,但温度仍是影响副乳房链球菌病发生、流行的主要因素。副乳房链球菌的致病分为急性感染和慢性感染两种,急性感染3~7 d内死亡率超过50%,慢性感染数周内只有少量死亡[10]。笔者分离到副乳房链球菌的季节是7月,水温为19 ℃,10日内患病个体死亡率约为75%。从患病个体肝、肾、脾等内脏分离出副乳房链球菌,且回归感染出现与自然感染发病相似的病征。此外,体表溃疡处也分离出罗氏假单胞菌、腐生葡萄球菌等继发感染的条件致病菌。
表1 部分鱼类感染副乳房链球菌的报道
Tab.1 Infection report of Streptococcus parauberis in fish
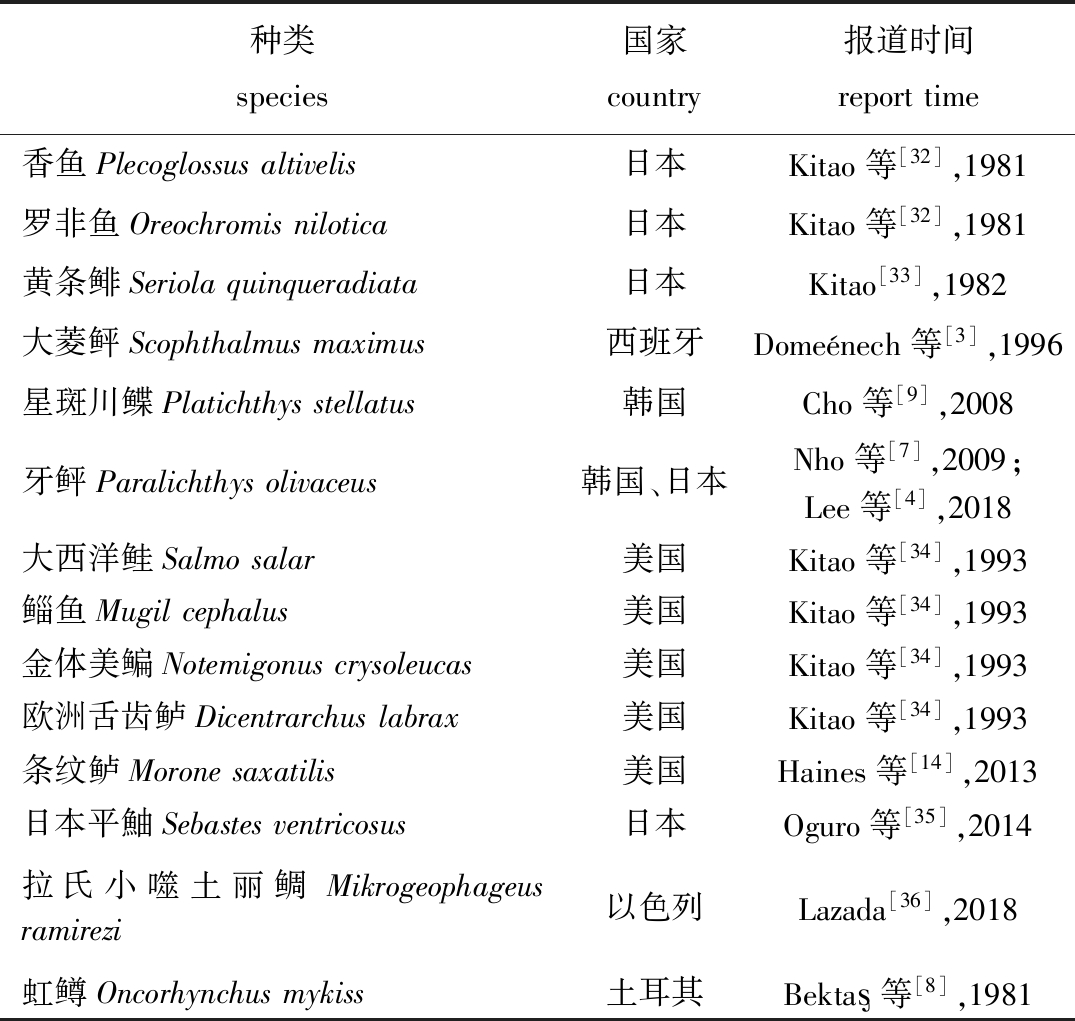
种类species国家country报道时间reporttime香鱼Plecoglossusaltivelis日本Kitao等[32],1981罗非鱼Oreochromisnilotica日本Kitao等[32],1981黄条鲱Seriolaquinqueradiata日本Kitao[33],1982大菱鲆Scophthalmusmaximus西班牙Domeénech等[3],1996星斑川鲽Platichthysstellatus韩国Cho等[9],2008牙鲆Paralichthysolivaceus韩国、日本Nho等[7],2009;Lee等[4],2018大西洋鲑Salmosalar美国Kitao等[34],1993鲻鱼Mugilcephalus美国Kitao等[34],1993金体美鳊Notemigonuscrysoleucas美国Kitao等[34],1993欧洲舌齿鲈Dicentrarchuslabrax美国Kitao等[34],1993条纹鲈Moronesaxatilis美国Haines等[14],2013日本平鮋Sebastesventricosus日本Oguro等[35],2014拉氏小噬土丽鲷Mikrogeophageusramirezi以色列Lazada[36],2018虹鳟Oncorhynchusmykiss土耳其Bekta 等[8],1981
2.2 临床症状
副乳房链球菌感染后表现为链球菌病的典型临床症状,病鱼一般出现食欲减退、不规则游动、体表发黑(图3C1)、眼球突出(图3A1)、败血性出血(内出血或兼有外源性出血,图3B1、A2、B2)、腹水腹胀(图3A3)、脾脏肾脏肿胀、内脏出血等症状[3,28-29,31]。组织病理变化主要表现在心脏和脑:心肌纤维被炎症细胞取代,单核淋巴细胞样细胞伴有结缔组织扩张和心肌细胞缺失,脑组织呈现严重的单核浸润和浓缩[29](图4),肝、肾、肌肉组织亦出现了不同程度的巨噬细胞浸润[36]。笔者分离到副乳房链球菌感染的自然发病大菱鲆,表现为眼球突出、下颌红肿,剖解发现其肝脏出血、脾脏肿大、肠道无食物或充满淡黄色积液。回归感染发现,不同浓度组个体反应不甚相同[15]:注射菌液浓度为6.3×109~6.3×1010CFU/mL时,受试鱼死亡率较高,14 d内累计死亡率超过90%,多数表现出腹部膨大红肿、肠壁稀薄透明状、脾脏肿大等急性感染临床症状,也有症状不明显的急性死亡病例;而6.3×107~6.3×108 CFU/mL浓度组则多表现出眼球突出、肌肉点状出血、内脏充血出血等慢性症状。
Kim等[29]应用荧光定量PCR监测副乳房链球菌感染牙鲆后主要攻击靶器官时,发现感染后第3天、第7天时,心脏及脑组织中细菌拷贝数最高,脾、肾、肠、肌肉等组织细菌拷贝数依次降低。该研究结果表明:感染后脑组织和心脏中细菌拷贝数 量最大,且组织出现明显病变,二者是副乳房链球菌感染的主要靶器官;感染后7 d内,细菌定植以心、脑为主逐渐变为全身系统感染;在感染初期,脾脏中细菌增殖快,是初期的最佳取样组织。
3 副乳房链球菌的致病机制
链球菌的毒力因子一般分为孔毒素、促进免疫逃避因子、黏附因子等几大类[37]。有研究表明,链球菌的致病力取决于细菌在宿主免疫细胞中的存活及避免被宿主细胞杀死而建立感染,以及诱导细胞凋亡的能力[38]。已有研究表明,某些毒力基因在海豚链球菌[39]、无乳链球菌[40]等细菌致病力方面发挥了重要作用,对菌株致病力强弱的判断可以通过检测其毒力因子加以辨别,而副乳房链球菌的相关研究报道较少,其致病机理与毒力基因关联度有待进一步研究。笔者结合本实验室检测副乳房链球菌分离株毒力基因携带情况,归纳总结了几种毒力因子的研究进展。

A1—患病虹鳟眼球突出;B1—患病虹鳟出血;C1—患病虹鳟体色发黑;A2—患病牙鲆眼及胸鳍出血;B2—患病牙鲆下颌充血发红;A3、B3—患病大菱鲆腹部膨大。
A1—exophthalmos of rainbow trout;B1—hemorrhage of rainbow trout;C1—darkening of the skin of rainbow trout;A2—hemorrhage of eyes and pectoral fin in olive flounder;B2—red swelling and congestion of jaw in olive flounder; A3 and B3—abdominal distension in turbot.
图3 虹鳟[8]、牙鲆[27]、大菱鲆副乳房链球菌感染典型症状
Fig.3 Typical symptoms of Streptococcus parauberis infection in rainbow trout Oncorhynchus mykiss[8], olive flounder Paralichy solivaceus[27] and turbot Scophthalmus maximus
3.1 荚膜多糖
荚膜是位于某些细菌细胞壁表面,包围整个菌体的特殊结构[41-42],因其具有抗原性,在血清型分型系统中可作为细菌鉴定的依据[43]。El Aamri等[44]认为,链球菌的荚膜在逃避宿主吞噬细胞的吞噬作用中起到了关键作用。副乳房链球菌亦不例外,其荚膜结构与抗血清杀伤活性、抗巨噬细胞吞噬能力直接相关[30]。此外,这些荚膜结构可抑制补体或抗体结合在细菌表面[45]。链球菌不同菌株有无荚膜导致毒力不同,有荚膜层的细菌类型毒力普遍高于无荚膜层的细菌类型,如格氏乳球菌KG-型(有荚膜层)毒力强于KG+型(无荚膜层)[25,37];海豚链球菌有荚膜的K+型菌株可感染牙鲆致病,而无荚膜的K-血清型则对牙鲆不具致病性[18]。
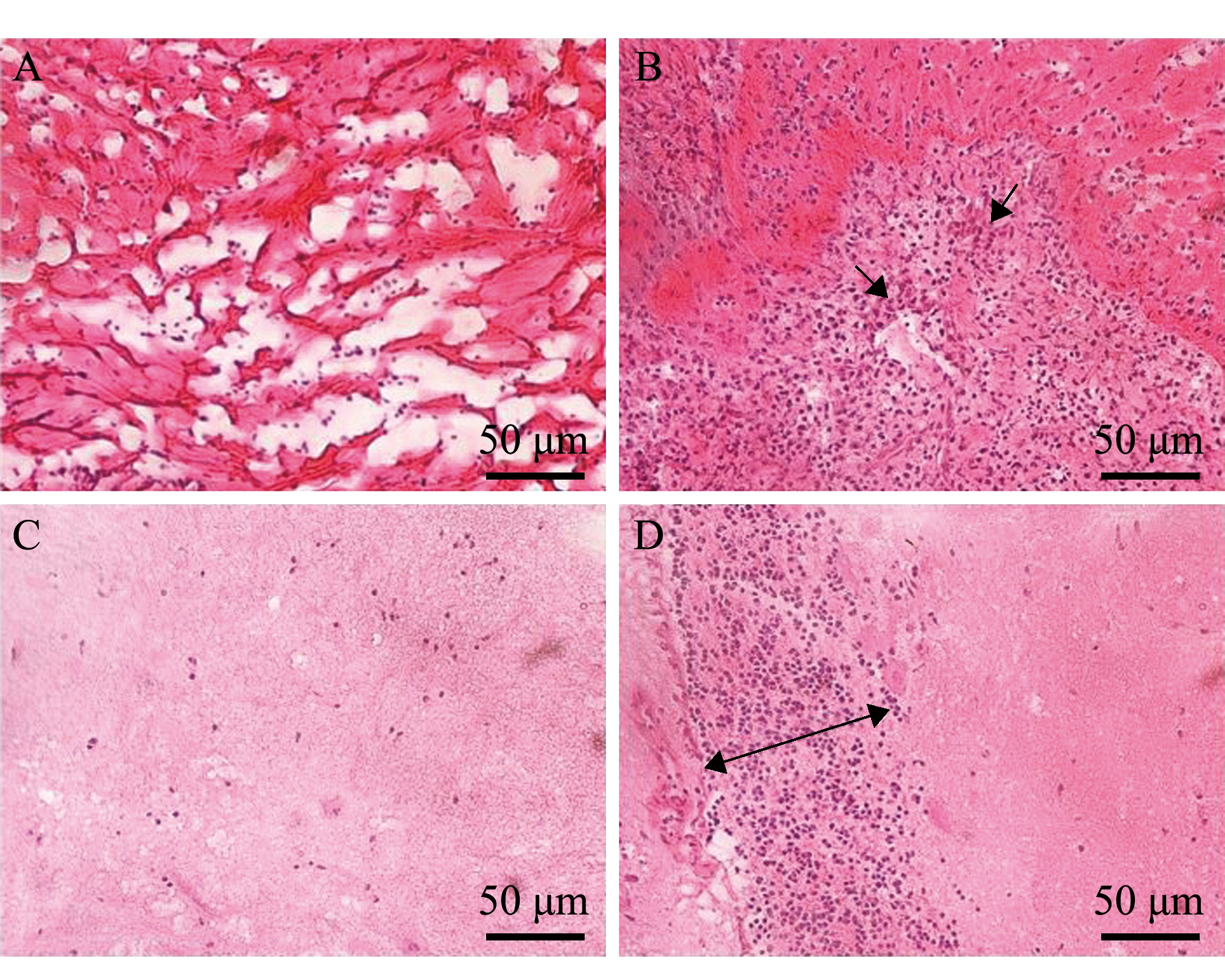
A—正常心脏组织切片;B—人工感染第7天时的心脏组织切片,箭头处示炎症区细胞浸润灶;C—正常脑组织切片;D—人工感染第7天时的脑组织,箭头处示炎症区细胞浸润灶。
A—normal heart tissue;B—infected heart tissue sampled 7 days post challenge,the arrow shows several small foci of celluar infiltrations in the inflammatory region;C—normal brain issue;D—infected brain tissue sampled 7 days post challenge,and the arrow shows several small foci of cellular infiltration.
图4 牙鲆副乳房链球菌感染后心、脑组织切片图[29]
Fig.4 Histopathological changes of the heart and brain after infection (H.E staining) in olive flounder Paralichys olivaceus[29]
链球菌的荚膜成分主要为荚膜多糖(polysaccharide capsules,CPS)[38],作为细菌的毒力因子,其结构差异对致病力至关重要,并在宿主免疫系统的逃逸、抗血清吸附、炎症及生物膜形成等方面发挥着重要作用[46-48],负责合成荚膜多糖的基因通常为聚集在染色体dexB和aliA间的一个完整转录单位[21]。革兰氏阳性菌荚膜多糖普遍存在一种特殊的盒装基因簇结构[49],即特异性基因位于中间区域,两侧是血清型高度同源的保守序列[50]。在研究副乳房链球菌不同血清型荚膜多糖结构时发现,其盒状基因簇结构由如下基因组成:在CPS保守区域的5′端,有一组包括lysR、cpsA、cpsB、cpsC和cpsD在内的5个调控基因,以及1个加工基因cpsE;在保守区域3′端有1个调控基因cpsQ和1个假设基因cpsR;CPS位点的中间可变区则包括糖基转移酶、乙酰转移酶、氨基转移酶等酶编码基因[20](图5)。应用荚膜多糖的结构差异可对副乳房链球菌不同血清型进行鉴别,其中,血清型Ⅰ与Ⅱ盒装基因簇结构相似度最高,Ⅰb与Ⅰc亚型因结构相同无法通过该基因结构差异有效区分[23]。
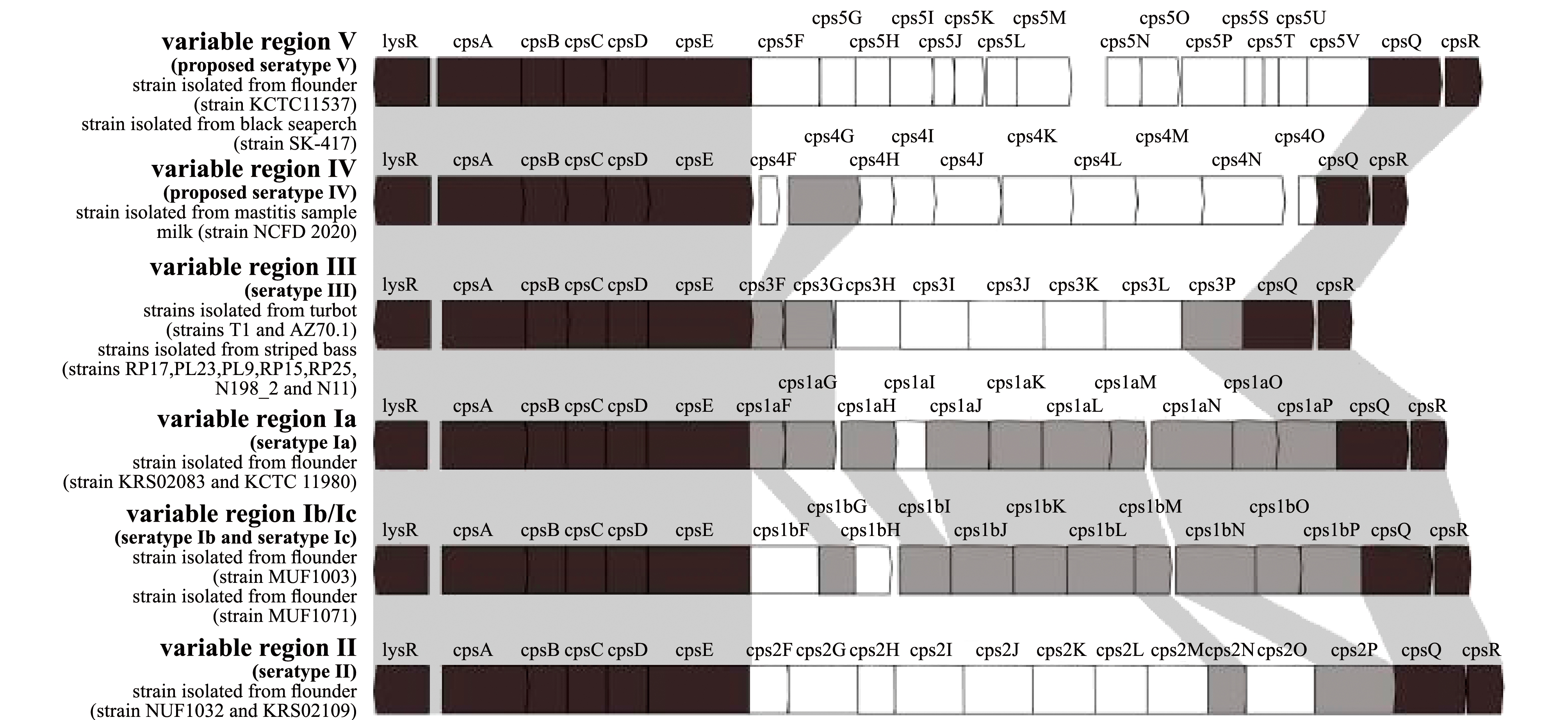
黑色区域为荚膜多糖保守区基因;灰色区域为存在于两个或两个以上血清型中的基因;白色区域为可变区基因。
Conserved regions of the cps locus of S.parauberis are shown in black color,genes in two or more serotypes are shown in gray color, and serotype-specific regions are shown in white.
图5 副乳房链球菌不同血清型荚膜多糖基因结构示意图[24]
Fig.5 Structure of loci cps gene of Streptococcus parauberis[24]
3.2 免疫相关蛋白
表面免疫原性蛋白(surface immunogenic protein,sip)是暴露于菌体表面的一类免疫相关蛋白,与细菌在宿主体内的黏附、定植有关[51]。最初是由Brodeur等[52]对人源无乳链球菌基因文库免疫学筛选时发现并命名。该蛋白存在于不同无乳链球菌血清型代表菌株中,且在对不同血清型致死剂量攻击的多种动物模型研究中均有较好的交叉免疫保护作用[53-54],因此,被列为研究无乳链球菌疫苗的良好候选靶抗原[55]。该基因具有高度的保守性,在147株牛源无乳链球菌中,仅发现4株LysM基因缺失[56];18株中国牛源分离株sip基因序列与另外13条GenBank下载序列间的核苷酸同源性在97.8%以上[57]。
某些链球菌细胞表面可产生免疫原性黏附蛋白(GBS immunogenic bacterial adhesion)基因bibA,以促进其在宿主细胞表面的吸附[58],且通过补体抑制剂C4b结合蛋白,对抵抗机体吞噬及毒理作用发挥具有关键作用,是一类新的革兰氏阳性表面黏附素[59],缺失bibA基因的菌株存活及抗吞噬作用明显降低[60-61]。
3.3 酶
透明质酸酶(hyaluronidase,hyl)属碱性糖蛋白类蛋白水解酶,可以特异性分解细胞外基质透明质酸(hyaluronidase acid),为细菌生长繁殖提供必需的能量并协助细菌在组织中散播[62-63],是细菌致病的重要毒力因子之一,又称扩散因子,尤其在链球菌等革兰氏阳性菌致病机制中发挥了重要作用[64]。研究表明,透明质酸酶B(hylB)作为无乳链球菌重要的毒力因子之一,是破坏血脑屏障诱发鱼源感染的重要因子[65],且不同来源的无乳链球菌hylB基因具有高度的保守性,通过对其蛋白表达及抗原性分析可为疫苗研制提供良好基础[66]。
超氧化物歧化酶(superoxide dismutase, SOD)是一类能够催化超氧离子通过歧化反应转化为氧气和过氧化氢的酶,其作为重要的抗氧化剂广泛存在于动植物与微生物中[67]。细菌中的SOD主要作用是中和超氧离子,从而抵御宿主活性物质的损害以达到在宿主体内存活、繁殖并感染宿主的目的[68]。
笔者曾检测到山东大菱鲆源副乳房链球菌分离株携带sip、bibA、hylB等酶和免疫相关蛋白类毒力基因[15],而SodA基因则是副乳房链球菌特异性检测的首选基因。由于副乳房链球菌毒力基因相关的研究报道较少,笔者结合自主分离株毒力基因携带情况阐述了上述基因在链球菌属其他菌种毒力发挥中的重要作用,鉴于其相对保守性及在其他致病菌疫苗研发方面的应用[51,60,69],研究者对副乳房链球菌上述毒力基因开展深入研究,将会为副乳房链球菌致病机制研究、基因疫苗的筛选与研发提供科学参考。
4 副乳房链球菌的检测方法
细菌的快速鉴定及准确分型对于分离株亲缘关系评估及病害防控均具有重要意义[20]。常规细菌鉴定技术是临床实验室对病原菌检测的最常用方法,主要包括分离培养、菌落特征观察、显微观察及生理生化测试等。对链球菌而言,该方法仅能初步确定检测目标,较难准确鉴别到种属水平[70-71]。商业化的鉴定系统由于种种缺陷亦难准确鉴别,对链球菌鉴定置信区间仅为79%~100%[14],尤其对副乳房链球菌的鉴别更有待商榷[13-14,72]。鉴于此,基于PCR技术的分子鉴定被广泛应用,已成为检测、鉴定细菌病原体的最常用技术。
4.1 生理生化特征检测技术
副乳房链球菌与乳房链球菌在生理生化、血清学等方面相似度较高,较难基于上述指标对二者有效区分[2]。Watts等[73]曾提出将β-葡萄糖醛酸苷酶作为区分二者的生化指标,但有学者对乳房链球菌、副乳房链球菌模式株及24株乳房链球菌疑似株进行鉴定时,发现上述菌株该指标的测试结果确有不同,但在限制性内切酶片段长度多态性(restriction fragment length polymorphism, RFLP)中进一步证实,乳房链球菌不同株产β-葡萄糖醛酸苷酶的结果存在差异,并不能将其作为鉴别依据[74]。副乳房链球菌、海豚链球菌、无乳链球菌部分生理生化特征见表2。
表2 副乳房链球菌、海豚链球菌、无乳链球菌生理生化特征
Tab.2 Physiological and biochemical characteristics of Streptococcus parauberis, S.agalactiac and S.iniae
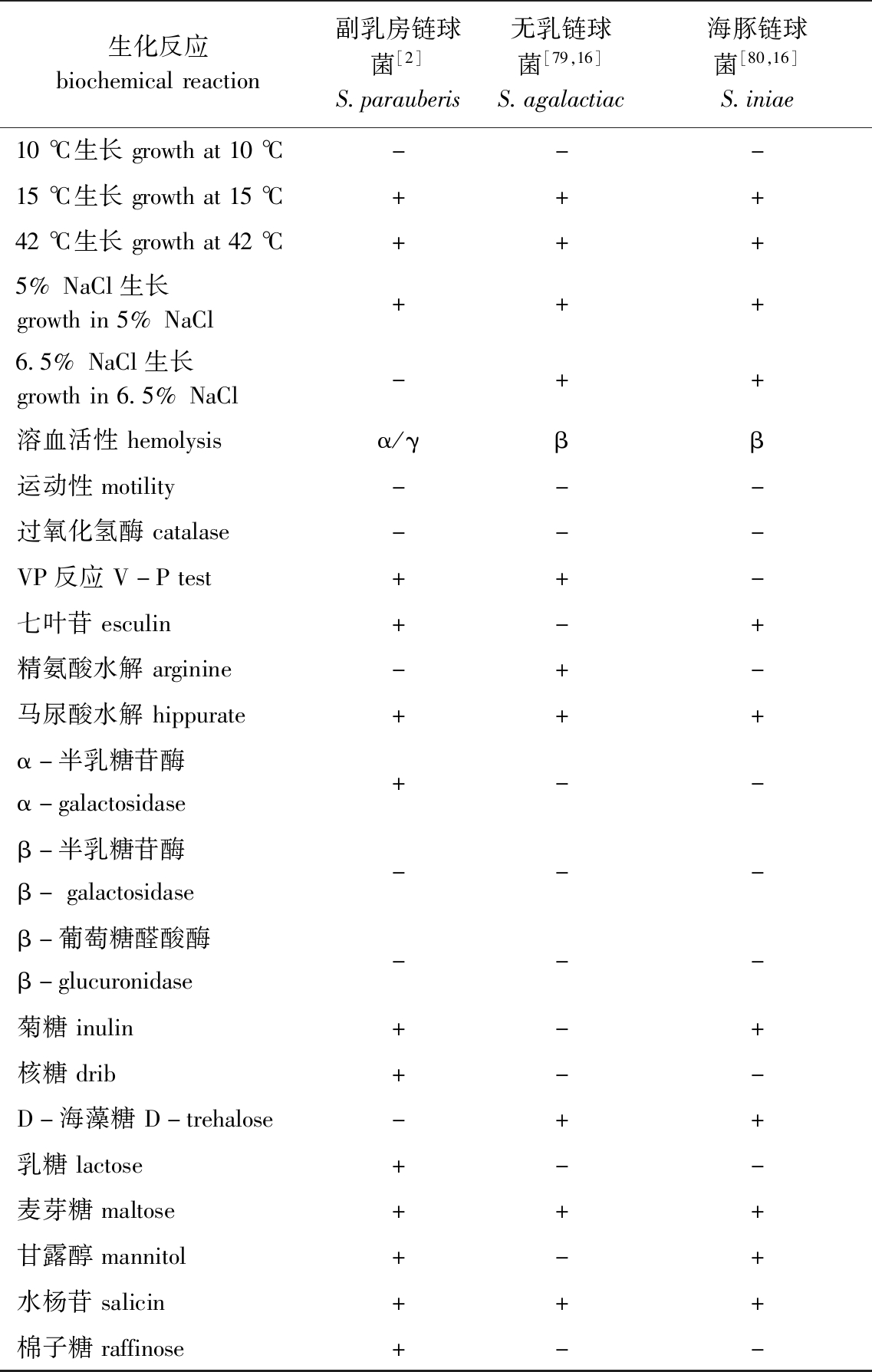
生化反应biochemicalreaction副乳房链球菌[2]S.parauberis无乳链球菌[79,16]S.agalactiac海豚链球菌[80,16]S.iniae10℃生长growthat10℃---15℃生长growthat15℃+++42℃生长growthat42℃+++5%NaCl生长growthin5%NaCl+++6.5%NaCl生长growthin6.5%NaCl-++溶血活性hemolysisα/γββ运动性motility---过氧化氢酶catalase---VP反应V-Ptest++-七叶苷esculin+-+精氨酸水解arginine-+-马尿酸水解hippurate+++α-半乳糖苷酶α-galactosidase+--β-半乳糖苷酶β-galactosidase---β-葡萄糖醛酸酶β-glucuronidase---菊糖inulin+-+核糖drib+--D-海藻糖D-trehalose-++乳糖lactose+--麦芽糖maltose+++甘露醇mannitol+-+水杨苷salicin+++棉子糖raffinose+--
4.2 基因检测技术
PCR技术实现了DNA预选区域扩增,直观呈现了分子水平差异,成为检测病原菌最常用的工具。16S rRNA是细菌鉴定最常用的基因[1,39],Bentley等[75-76]认为,根据16S rRNA V2区序列变异可实现链球菌属31种病原菌的有效鉴别,包括乳房链球菌和副乳房链球菌。Hassan等[1]根据16S-23S rRNA特异性区域及间隔区构建的引物,扩增乳房链球菌和副乳房链球菌分别得到445、884 bp产物。目前,用于鉴定副乳房链球菌的候选基因主要有23S rRNA(Spa2152/Spa2870)[13]、乳酸氧化酶lctO(LOX-1/LOX-2)[13]、伴侣蛋白HSP 60(cpn60F/cpn60R)[15]、超氧化物歧化酶A(SodAF/SodAR)[15]等,常规PCR检测灵敏度一般为1 pg,荧光定量PCR灵敏度为0.1 pg[77]。Kitao[33]基于23S rRNA、16S rRNA、gyrB设计了多重PCR,可检测褐牙鲆3种重要的病原菌——副乳房链球菌、海豚链球菌、迟缓爱德华氏菌,分别得到718、300、415 bp的产物,最高检出限灵敏度为1 ng。Nguyen等[78]以gyrB基因序列为靶点,采用TaqMan探针法开发了副乳房链球菌的实时荧光定量PCR检测方法,该方法灵敏度高、变异系数低,与亲缘关系较近的海豚链球菌亦无交叉反应,可用于副乳房链球菌的定量检测。
4.3 全基因组测序技术
通过对细菌全基因组DNA测序、拼接、组装,可以获得完整的基因组信息。该技术成本较高,但获得的生物信息为深入挖掘基因功能,以及致病机制与免疫机制研究提供了可能[81]。Liu等[82]获得了牛源副乳房链球菌株SP-llh全基因组序列,包含2 522 235 bp碱基及2 620个编码蛋白的基因。Lee等[4]获得了牙鲆源多重耐药副乳房链球菌株SPOF3K的全基因组序列,该序列包含2 128 740 bp的圆形染色体及23 538 bp质粒,分别包含2 123和24个编码蛋白的基因。Halnes等[83]测定了7株野生条纹鲈鱼源副乳房链球菌全基因组序列信息,片段长度为1 999 273~2 031 135 bp,编码蛋白的基因数为1 972~2 057,G+C含量为35.5%~39.0%。
5 副乳房链球菌的耐药性
5.1 药物敏感性
目前,对链球菌的防治主要以抗菌药物为主,病原菌对抗菌药物的耐受程度直接决定了药物防治效果,而抗菌药物的频繁使用又增加了病原菌对该药物或同类药物的耐药性。近年来,众多学者对不同国家或地区分离到的鱼源副乳房链球菌株开展了耐药性测试。研究表明,韩国牙鲆源分离株对氨苄西林、阿莫西林、多西环素、复方新诺明、土霉素、庆大霉素、红霉素等药物存在多重耐药[4];土耳其虹鳟源分离株对依诺沙星、土霉素和氯霉素耐药,对多西环素、氟苯尼考、恩诺沙星、万古霉素、红霉素、青霉素敏感[8];美国野生条纹鲈鱼源分离株对复方新诺明耐药,对磺胺类药物普遍中介,对四环素、万古霉素、红霉素、青霉素敏感[14]。不同分离株对药物的耐受程度与该地区抗生素类药物的使用频率与使用量有关,分离株的耐药表现提示,在该病的药物防治中应更加科学且和针对性,谨防多重耐药加剧。此外,多数副乳房链球菌分离株对青霉素敏感,证实了Cameron等[84]关于环境链球菌对β-内酰胺类药物敏感的论断。
5.2 耐药基因检测
细菌耐药基因检测是研究细菌耐药机制的重要手段。Zhang等[85]报道了16株乳房链球菌和11株副乳房链球菌对大环内酯类(mefA、ermA、ermB、ermC)、四环素类(tetO、tetL、tetM、tetK、tetS)、氨基糖苷类(aacA-aphD)、β-内酰胺类(blaZ)、黏附分子(sua)、纤维酶原激活物(pauA/skc)、表面脱氢酶(gapC)、乳铁蛋白结合蛋白(lbp)、CAMP因子(cfu)和透明质酸酶(hasA、hasB、hasC)等10类耐药基因及毒力相关基因的携带情况。药物敏感性检测结果对比发现,尽管90.9%的副乳房链球菌分离株对红霉素耐药,但其耐药基因ermB的携带率仅为27.3%,耐药表现与耐药基因检测结果不完全一致,这种差异可能与耐药机制有关。链球菌耐药性的产生主要有两种机制,即耐药质粒的转移或核糖体的保护[77],上述差异可能与抗生素物质的外排或核糖体差异有关[86]。
6 副乳房链球菌的其他防治措施
6.1 疫苗
疫苗不仅是防控养殖动物发病的有效途径,也是保障水产品质量及环境安全的重要途径之一。鱼源副乳房链球菌疫苗主要是全细胞灭活疫苗,传统福尔马林灭活全细胞疫苗免疫保护作用明显[87-88],相对免疫保护率可达100%。但添加聚D,L-丙交酯-羟基乙酸(PLGA)包埋后的灭活疫苗效力更长,免疫保护率更高,且成本较低,适应性免疫应答和免疫持续时间是该类疫苗商业化应用前的研究方向之一[89]。副乳房链球菌疫苗的接种方式一般为腹腔注射[90],尽管口服方式可以消除注射方式劳动量大、对鱼体有应激或损伤等弊端,更适用于水生动物,但该方式受摄食行为、养殖环境影响较大。Kim等[91]采用海藻酸盐离子凝胶包埋法制作了福尔马林灭活口服疫苗,对牙鲆幼鱼免疫保护率更高,适用于仔鱼阶段免疫。此外,有研究团队对副乳房链球菌联合疫苗开展了相关研究,如海豚链球菌与副乳房链球菌血清型Ⅰ、Ⅱ[92],迟缓爱德华氏菌、海豚链球菌与副乳房链球菌[93],迟缓爱德华氏菌运动与非运动型、海豚链球菌、副乳房链球菌不同血清型[94]等三联、五联疫苗,均对此类细菌病有一定的免疫保护作用。
6.1 噬菌体
噬菌体是一类细菌性病毒,专以细菌为宿主,能引起宿主菌的裂解,从而达到抗菌效果,被认为是极具潜力的抗生素替代品。与传统抗生素相比,噬菌体具有专一性强、可自我复制增殖、来源丰富、筛选方便等优势,无论宿主菌药物敏感性如何,噬菌体均可感染并杀死宿主菌,是治疗细菌感染的新选择。自20世纪80年代学者证实了噬菌体的体外杀菌作用后,噬菌体陆续在黄条鰤格乳球菌病、香鱼冷水病、大西洋鲑杀鲑气单胞菌病、斑节对虾发光病、刺参弧菌病等细菌感染中尝试应用[95]。Kwon等[96]对一株副乳房链球菌噬菌体进行研究,发现该噬菌体对副乳房链球菌专一性较高,在55株测试菌株中,63.6%对该噬菌体敏感,无论采样分离季节和菌株耐药性如何,均能有效降低副乳房链球菌感染发病率,并在一定程度上提高水产动物生长性能,是一种环境友好型抗生素替代品。
7 存在问题及展望
副乳房链球菌是一种分布广、致病力强、危害严重的细菌性病原。尽管中国鱼源副乳房链球菌病并未大面积暴发流行,但该病曾给日韩等亚洲国家鱼类养殖造成了巨大经济损失,对其开展深入研究对中国该病的防控具有重要意义。近年来,虽然国外众多学者对其开展过深入研究,但其流行病学、传播途径、致病机制、分子分型等内容尚不明确、行之有效的防控措施亦未完全建立。未来可在以下几方面重点开展深入研究。
1)加强流行病学研究。要从源头解决副乳房链球菌对鱼类的影响,必须加强对其流行病学的研究。该菌既能感染牛引发乳房炎,又能感染鱼类引发链球菌病,尽管牛源与鱼源分离株分子差异已有报道,但不同来源菌株间的遗传进化关系、流行传播规律尚待进一步研究,鱼类菌株是否来源于牛源,菌株是否存在跨种传播亦需明确。链球菌病属中国水产养殖三类疫病,探明其流行、发生规律,对由该菌引起的链球菌病防治将起到有效预警。
2)重点开展致病机制研究。目前,副乳房链球菌的毒力基因相关研究鲜有报道,致病机制尚待进一步明确,开展此方面的研究对链球菌病防控具有重要意义。与链球菌属其他致病菌相似,副乳房链球菌感染可能是由细菌突破血脑屏障或单核、巨噬细胞携带入血或进入神经系统进而诱发,但其感染模式与致病机制尚未明确;基于荚膜多糖结构差异可将副乳房链球菌分为不同血清型,除物种来源外,鱼源亦可细分,荚膜多糖已被证实是其重要的毒力因子,目前亟需确认其他毒力因子,为深入研究其致病机制、探求基于分子生物学的有效防控策略奠定基础。
3)科学筛选防治药物。目前,药物仍然是副乳房链球菌病防控的主要手段,但抗生素类药物的大量使用不仅对环境造成不利影响,还会导致细菌耐药甚至产生多重耐药。不同国家的副乳房链球菌分离株已出现了一定程度的耐药,如何科学筛选敏感药物、避免多重耐药,寻求抗生素替代物尚需试验研究。在山东省大菱鲆养殖中,已有企业尝试无抗化养殖模式,该模式通过提高水质、控制密度、加强管理、特殊时期密切防控等手段控制零抗生素添加,从源头解决了抗生素使用的问题,在一定范围内可复制推广。
4)加大疫苗研发力度。链球菌疫苗研发具有一定基础,但临床效果有待进一步验证,当前阶段,研发安全高效的副乳房链球菌疫苗,是防控该病的重要手段。目前,疫苗在日本牙鲆源副乳房链球菌病的防控中取得了一定的效果,但疫苗的免疫保护率不能覆盖整个鱼类养殖周期,且常规腹腔注射劳动量大、易造成损伤或应激,因此,亟需加强口服或浸泡疫苗的研制。DNA疫苗是未来疫苗研制的方向,但在环境选择压力下,人工干预株是否存在传播或突变可能仍需进一步研究。
[1] HASSAN A A,KHAN I U,ABDULMAWJOOD A,et al.Evaluation of PCR methods for rapid identification and differentiation of Streptococcus uberis and Streptococcus parauberis[J].Journal of Clinical Microbiology,2011,39(4):1618-1621.
[2] WILLIAMS A M,COLLINS M D.Molecular taxonomic studies on Streptococcus uberis types Ⅰ and Ⅱ.Description of Streptococcus parauberis sp.nov.[J].Journal of Applied Bacteriology,1990,68(5):485-490.
[3] DOME NECH A,DERENA
NECH A,DERENA ANDEZ-GARAYZ
ANDEZ-GARAYZ BAL J F,PASCUAL C,et al.Streptococcosis in cultured turbot,Scopthalmus maximus (L.),associated with Streptococcus parauberis[J].Journal of Fish Diseases,1996,19(1):33-38.
BAL J F,PASCUAL C,et al.Streptococcosis in cultured turbot,Scopthalmus maximus (L.),associated with Streptococcus parauberis[J].Journal of Fish Diseases,1996,19(1):33-38.
[4] LEE Y,NGUYEN T L,KIM A,et al.Complete genome sequence of multiple-antibiotic-resistant Streptococcus parauberis strain SPOF3K,isolated from diseased olive flounder(Paralichthys olivaceus)[J].Genome Announcements,2018,6(17):e00248-18.
[5] 李新圃,罗金印,杨峰,等.6株牛源副乳房链球菌的分离和鉴定[J].中国兽医学报,2016,36(10):1710-1713.
LI X P,LUO J Y,YANG F,et al.Isolation and identification of six Streptococcus parauberis from cow[J].Chinese Journal of Veterinary Medicine,2016,36(10):1710-1713.(in Chinese)
[6] 任梅渗,王印,杨泽晓,等.一株副乳房链球菌的分离鉴定及生物学分析[J].浙江农业学报,2016,28(5):758-762.
REN M S,WANG Y,YANG Z X,et al.Isolation,identification and biological characteristics analysis of Streptococcus parauberis[J].Acta Agriculturae Zhejiangensis,2016,28(5):758-762.(in Chinese)
[7] NHO S W,SHIN G W,PARK S B,et al.Phenotypic characteristics of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus)[J].FEMS Microbiology Letters,2009,293(1):20-27.
[8] BEKTA Z H,UÇAR F B,SAVA
Z H,UÇAR F B,SAVA ER S.Isolation and identification of Streptococcus parauberis from freshwater fish in Turkey[J].Journal of Limnology and Freshwater Fisheries Research,2017,3(3):175-182.
ER S.Isolation and identification of Streptococcus parauberis from freshwater fish in Turkey[J].Journal of Limnology and Freshwater Fisheries Research,2017,3(3):175-182.
[9] CHO M Y,KIM M S,CHOI H J,et al.Isolation of Streptococcus parauberis from starry flounder,Platichthys stellatus Pallas[J].Journal of Fish Pathology,2008,21(3):209-217.
[10] PARK Y K,NHO S W,SHIN G W,et al.Antibiotic susceptibility and resistance of Streptococcus iniae and Streptococcus parauberis isolated from olive flounder (Paralichthys olivaceus)[J].Veterinary Microbiology,2009,136(1/2):76-81.
[11] ELDAR A,GHITTINO C.Lactococcus garvieae and Streptococcus iniae infections in rainbow trout Oncorhynchus mykiss:similar,but different diseases[J].Diseases of Aquatic Organisms,1999,36(3):227-231.
[12] HOSHINA T,SANO T,MORIMOTO Y.A Streptococcus pathogenic to fish[J].Journal of Tokyo University of Fisheries,1958,44:57-68.
[13] MATA A I,GIBELLO A,CASAMAYOR A,et al.Multiplex PCR assay for detection of bacterial pathogens associated with warm-water streptococcosis in fish[J].Applied and Environmental Microbiology,2004,70(5):3183-3187.
[14] HAINES A T,GAUTHIER D E,NEBERGALL E E,et al.First report of Streptococcus parauberis in wild finfish from North America[J].Veterinary Microbiology,2013,166(1/2):270-275.
[15] 王鹤,李战军,黄华,等.一株鱼源副乳房链球菌的分离鉴定及其分子特性[J].大连海洋大学学报,2021,36(4):563-572.
WANG H,LI Z J,HUANG H,et al.Isolation,identification and molecular characteristic of Streptococcus parauberis from cultured turbot Scophthalmus maximus[J].Journal of Dalian Ocean University,2021,36(4):563-572.(in Chinese)
[16] 罗晓雯,李莉,朱永肖,等.鱼类海豚链球菌病研究进展[J].水产科学,2018,37(6):847-854.
LUO X W,LI L,ZHU Y X,et al.Research progress on diseases caused by pathogen Streptococcus iniae in fishes:a review[J].Fishes Science,2018,37(6):847-854.(in Chinese)
[17] NGUYEN H T,KANAI K.Selective agars for the isolation of Streptococcus iniae from Japanese flounder,Paralichthys olivaceus,and its cultural environment[J].Journal of Applied Microbiology,1999,86(5):769-776.
[18] KANAI K,YAMADA M,MENG F,et al.Serological differentiation of Streptococcus parauberis strains isolated from cultured Japanese flounder in Japan[J].Fish Pathology,2009,44(1):33-39.
[19] KANAI K,TU C,KATAYAMA N,et al.Existence of subserotypes in Streptococcus parauberis serotype I[J].Fish Pathology,2015,50(2):75-80.
[20] YOLANDA T C,CLARA F  ,YSABEL S Y.Proteomic and molecular fingerprinting for identification and tracking of fish pathogenic Streptococcus[J].Aquaculture,2019,498:322-334.
,YSABEL S Y.Proteomic and molecular fingerprinting for identification and tracking of fish pathogenic Streptococcus[J].Aquaculture,2019,498:322-334.
[21] TU C,SUGA K,KANAI K.Structure of genetic loci for capsular polysaccharide biosynthesis in Streptococcus parauberis isolated from Japanese flounder[J].Fish Pathology,2015,50(4):192-199.
[22] TU C,SUGA K,KANAI K.A multiplex PCR assay for different-iation of Streptococcus parauberis serotypes[J].Fish Pathology,2015,50(4):213-215.
[23] TORRES-CORRAL Y,SANTOS Y.Comparative genomics of Streptococcus parauberis:new target for molecular identification of serotype [J].Applied Microbiology and Biotechnology,2020,104(14):6211-6222.
[24] HAN S Y,KANG B K,KANG B J,et al.Prevalence and different characteristics of two serotypes of Streptococcus parauberis isolated from the farmed olive flounder,Paralichthys olivaceus (Temminck and Schlegel),in Korea[J].Journal of Fish Diseases,2011,34(10):731-739.
[25] PARK J Y,BIRHANU B T,LEE S J,et al.Pharmacodynamics of amoxicillin against field isolates of Streptococcus parauberis from olive flounder (Paralichthys olivaceus)[J].Aquaculture Research,2018,49(2):1060-1071.
[26] FERN NDEZ-NO I C,BÖHME K,CALO-MATA P,et al.Isolation and characterization of Streptococcus parauberis from vacuum-packaging refrigerated seafood products[J].Food Microbiology,2012,30(1):91-97.
NDEZ-NO I C,BÖHME K,CALO-MATA P,et al.Isolation and characterization of Streptococcus parauberis from vacuum-packaging refrigerated seafood products[J].Food Microbiology,2012,30(1):91-97.
[27] KIM J H,GOMEZ D K,BAECK G W,et al.Pathogenicity of Streptococcus parauberis to olive flounder Paralichthys olivaceus[J].Fish Pathology,2006,41(4):171-173.
[28] WON K M,CHO M Y,PARK M A,et al.Pathological characteristics of olive flounder Paralichthys olivaceus experimentally infected with Streptococcus parauberis[J].Fisheries Science,2010,76(6):991-998.
[29] KIM H,KIM A,KIM S M,et al.Genome based quantification of Streptococcus parauberis in multiple organs of infected olive flounder (Paralichthys olivaceus)[J].Genes and Genom,2017,39(8):897-902.
[30] HWANG S D,WOO S H,KIM S H,et al.Immunomodulatory characteristics of Streptococcus parauberis isolated from infected olive flounder,Paralichthys olivaceus[J].Journal of Fish Pathology,2008,21(3):157-166.
[31] MORI K,FUKUDA Y,TOGO A,et al.Practical inoculation site for experimental infection of Japanese flounder Paralichthys olivaceus with Streptococcus parauberis[J].Fish Pathology,2010,45(1):37-42.
[32] KITAO T,AOKI T,SAKOH R.Epizootic caused by β-haemoltytic Streptococcus species in cultured freshwater fish[J].Fish Pathology,1981,15(3/4):301-307.
[33] KITAO T.The methods for detection of Streptococcus sp.,causative bacteria of streptococcal disease of cultured yellowtail (Seriola quinqueradiata)[J].Fish Pathology,1982,17(1):17-26.
[34] KITAO K,STREPTOCOCCA L,INFECTION S.Bacterial disease of fish[M].Oxford:Blackwell Scientific Publications,1993:196-210.
[35] OGURO K,YAMANE J,YAMAMOTO T,et al.Draft genome sequence of Streptococcus parauberis strain SK-417,isolated from diseased Sebastes ventricosus in Kagoshima,Japan[J].Genome Announc,2014,2(3):e00453-e00414.
[36] LAZADA C C,FRIDMAN S,SINAI T,et al.First report of Streptococcus parauberis in a cultured freshwater ornamental fish,the ram cichlid Mikrogeophagus ramirezi (Myers & Harry,1948)[J].Journal of Fish Diseases,2018,41(1):161-164.
[37] 李晓雪.Sip蛋白高亲和性噬菌体介导的牛源无乳链球菌间接ELISA方法的建立[D].哈尔滨:东北农业大学,2015.
LI X X.An indirect ELISA method established for detecting Streptococcus agalactiae mediated by high affinity phages with Sip protein[D].Harbin:Northeast Agricultural University,2015.(in Chinese)
[38] YOSHIDA T,ENDO M,SAKAI M,et al.A cell capsule with possible involvement in resistance to opsonophagocytosis in Enterococcus seriolicida isolated from yellowtail Seriola quinqueradiata[J].Disease of Aquatic Organisms,1997,29(3):233-235.
[39] 邓梦玲,耿毅,刘丹,等.西伯利亚鲟海豚链球菌的分离鉴定及毒力基因检测[J].水产学报,2015,39(1):127-135.
DENG M L,GENG Y,LIU D,et al.Isolation,identification and detection of virulence genes of Streptococcus iniae from Acipenser baerii[J].Journal of Fisheries of China,2015,39(1):127-135.(in Chinese)
[40] 王巧煌.福建红罗非鱼源无乳链球菌的分离鉴定及其分子特征[J].大连海洋大学学报,2019,34(1):63-69.
WANG Q H.Isolation,identification,and molecular characteristics of Streptococcus agalactiae from cultured red tilapia in Fujian Province[J].Journal of Dalian Ocean University,2019,34(1):63-69.(in Chinese)
[41] 王楷宬,陆承平,范伟兴.细菌荚膜多糖[J].微生物学报,2011,51(12):1578-1584.
WANG K C,LU C P,FAN W X.Bacterial capsular polysaccharide:a review[J].Acta Microbiologica Sinica,2011,51(12):1578-1584.(in Chinese)
[42] 李天一,王英锋,张玫.细菌荚膜多糖的研究进展[J].中国人兽共患病学报,2015,31(12):1171-1176.
LI T Y,WANG Y F,ZHANG M.Research progress on bacterial capsular polysaccharide[J].Chinese Journal of Zoonoses,2015,31(12):1171-1176.(in Chinese)
[43] 陆承平.兽医微生物学[M].4版.北京:中国农业出版社,2007:15-16.
LU C P.Veterinary microbiology[M].4th edition.Beijing:China Agriculture Press,2007:15-16.(in Chinese)
[44] EL AAMRI F,CABALLERO M J,REAL F,et al.Streptococcus iniae in gilthead seabream (Sparus aurata L.) and red porgy (Pagrus pagrus L.):ultrastructural analysis[J].Veterinary Pathology,2015,52(1):209-212.
[45] SHUTOU K,KANAI K,YOSHIKOSHI K.Virulence attenuation of capsular polysaccharide-deleted mutants of Streptococcus iniae in Japanese flounder Paralichthys olivaceus[J].Fish Pathology,2007,42(1):41-48.
[46] HANCOCK L E,GILMORE M S.The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall[J].Proceedings of the National Academy of Sciences of the United States of America,1999,99(3):1574-1579.
[47] OKURA M,TAKAMATSU D,MARUYAMA F,et al.Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis:potential mechanisms for generation of capsular variation[J].Applied and Environmental Microbiology,2013,79(8):2796-2806.
[48] ROCHAT T,FUJIWARA-NAGATA E,CALVEZ S,et al.Genomic characterization of Flavobacterium psychrophilum serotypes and development of a multiplex PCR-based serotyping scheme[J].Frontiers in Microbiology,2017,8:01752.
[49] MAVROIDI A,AANENSEN D M,GODOY D,et al.Genetic relatedness of the Streptococcus pneumoniae capsular biosynthetic loci[J].Journal of Bacteriology,2007,189(21):7841-7855.
[50] WESSELS M R.Biology of streptococcal capsular polysaccharides[J].Journal of Applied Microbiology,1997,83(sup1):20S-31S.
[51] MAIONE D,MARGARIT I,RINAUDO C D,et al.Identification of a universal group B streptococcus vaccine by multiple genome screen[J].Science,2005,309(5731):148-150.
[52] BRODEUR B R,BOYER M,CHARLEBOIS I,et al.Identification of group B streptococcal Sip protein,which elicits cross-protective immunity[J].Infection and Immunity,2000,68(10):5610-5618.
[53] 于丽华,李慎涛,岳丽琴,等.B族链球菌表面蛋白Sip的制备及其免疫原性的研究[J].中华微生物学和免疫学杂志,2007,27(6):579-580.
YU L H,LI S T,YUE L Q,et al.Preparation of the surface protein Sip of group B streptococcus and its immunogenicity[J].Chinese Journal of Microbiology and Immunology,2007,27(6):579-580.(in Chinese)
[54] 黎炯,叶星,可小丽,等.罗非鱼无乳链球菌Sip基因的克隆、表达及免疫原性分析[J].水生生物学报,2012,36(4):626-633.
LI J,YE X,KE X L,et al.Cloning,expression and immunogenicity analysis of surface immunogenic protein (Sip) of tilapia Streptococcus agalactiae[J].Acta Hydrobiologica Sinica,2012,36(4):626-633.(in Chinese)
[55] VIDOV B,CHOT
B,CHOT R M,GOD
R M,GOD NY A.N-terminal anchor in surface immunogenic protein of Streptococcus agalactiae and its influence on immunity elicitation[J].Folia Microbiologica,2009,54(2):161-166.
NY A.N-terminal anchor in surface immunogenic protein of Streptococcus agalactiae and its influence on immunity elicitation[J].Folia Microbiologica,2009,54(2):161-166.
[56] 杨学云,王蒴,吴建勇,等.无乳链球菌Sip基因的克隆与原核表达[J].草食家畜,2016(1):36-41.
YANG X Y,WANG S,WU J Y,et al.Cloning and prokaryotic expression of Streptococcus agalactiae Sip gene[J].Grass-Feeding Livestock,2016(1):36-41.(in Chinese)
[57] 王旭荣,张世栋,杨峰,等.Ⅰa型和Ⅱ型牛源无乳链球菌Sip基因的遗传进化分析[J].中国兽医科学,2012,42(9):888-894.
WANG X R,ZHANG S D,YANG F,et al.Genetic evolution analysis of Sip gene of serotypes Ⅰa and Ⅱ of Streptococcus agalactiae isolates from cattle[J].Chinese Veterinary Science,2012,42(9):888-894.(in Chinese)
[58] 常瑞祥.奶牛隐性乳房炎无乳链球菌的分离鉴定及其BibA重组蛋白的表达与纯化[D].兰州:中国农业科学院兰州畜牧与兽药研究所,2014.
CHANG R X.Isolation,identification of the Streptococcus agalactiae from bovine subclinical mastitis and expression,purification of the BibA recombinant protein[D].Lanzhou:Chinese Academy of Agricultural Sciences,2014.(in Chinese)
[59] MANNE K,CHATTOPADHYAY D,AGARWAL V,et al.Novel structure of the N-terminal helical domain of BibA,a group B streptococcus immunogenic bacterial adhesin[J].Acta Crystallographica Section D,2020,76(8):759-770.
[60] SANTI I,MAIONE D,GALEOTTI C I,et al.BibA induces opsonizing antibodies conferring in vivo protection against group B Streptococcus[J].The Journal of Infectious Diseases,2009,200(4):564-570.
[61] DOS SANTOS N F B,DA SILVA L R,COSTA F J M D,et al.Immunization with a recombinant BibA surface protein confers immunity and protects mice against group B Streptococcus (GBS) vaginal colonization[J].Vaccine,2020,38(33):5286-5296.
[62] STARR C R,ENGLEBERG N C.Role of hyaluronidase in subcutaneous spread and growth of group A Streptococcus[J].Infection and Immunity,2006,74(1) :40-48.
[63] 黄源春,钱元恕,焦晓阳.透明质酸酶在细菌致病机制中的作用[J].中国感染与化疗杂志,2008,8(3):235-238.
HUANG Y C,QIAN Y S,JIAO X Y.Role of hyaluronidase in bacterial pathogenesis[J].Chinese Journal of Infection and Chemotherapy,2008,8(3):235-238.(in Chinese)
[64] ZWIJNENBURG P J G,VAN DER POLL T,FLORQUIN S,et al.Experimental pneumococcal meningitis in mice:a model of intranasal infection[J].The Journal of Infectious Diseases,2001,183(7):1143-1146.
[65] 罗素,马可,王兆飞,等.无乳链球菌透明质酸酶的可溶性表达及其对小鼠的致病作用[J].中国兽医科学,2016,46(8):997-1002.
LUO S,MA K,WANG Z F,et al.Prokaryotic expression of hyaluronidase from Streptococcus agalactiae and its pathogenicity in mice[J].Chinese Veterinary Science,2016,46(8):997-1002.(in Chinese)
[66] 王兆飞,郭长明,陆承平,等.鱼源无乳链球菌透明质酸酶的表达及抗原性分析[J].畜牧与兽医,2014,46(1):24-27.
WANG Z F,GUO C M,LU C P,et al.Expression and antigenicity analysis of hyaluronidase (hylB) of piscine Streptococcus agalactiae[J].Animal Husbandry & Veterinary Medicine,2014,46(1):24-27.(in Chinese)
[67] 唐宇龙.猪链球菌2型Sortases、CcpA和SodA功能与致病能力研究[D].杭州:浙江大学,2012.
TANG Y L.Functional analysis of Sortases,CcpA and SodA of Streptococcus suis type 2 in relation to pathogenicity[D].Hangzhou:Zhejiang University,2012.(in Chinese)
[68] BRUNO-B RCENA J M,AZC
RCENA J M,AZC RATE-PERIL M A,KLAENHAMMER T R,et al.Marker-free chromosomal integration of the manganese superoxide dismutase gene (SodA) from Streptococcus thermophilus into Lactobacillus gasseri[J].FEMS Microbiology Letters,2005,246(1):91-101.
RATE-PERIL M A,KLAENHAMMER T R,et al.Marker-free chromosomal integration of the manganese superoxide dismutase gene (SodA) from Streptococcus thermophilus into Lactobacillus gasseri[J].FEMS Microbiology Letters,2005,246(1):91-101.
[69] 袁玉梅,石存斌,陶家发,等.罗非鱼无乳链球菌Sip-Pgk融合蛋白乳酸菌口服疫苗制备及其免疫效果的研究[J].南方水产科学,2019,15(6):9-18.
YUAN Y M,SHI C B,TAO J F,et al.Preparation and immunogenicity of Lactococcus lactis vaccine expressing Sip-Pgk fusion protein of Streptococcus agalactiae isolated from tilapia[J].South China Fisheries Science,2019,15(6):9-18.(in Chinese)
[70] TOMA L,Di DOMENICO E G,PRIGNANO G,et al.Comment on “intravitreal ampicillin sodium for antibiotic-resistant endophthalmitis:Streptococcus uberis First Human Intraocular Infection Report”[J].Journal of Ophthalmology,2014:395480.
[71] FACKLAM R R,ELLIOTT J A.Identification,classification,and clinical relevance of catalase-negative,gram-positive cocci,excluding the streptococci and enterococci[J].Clinical Microbiology Reviews,1995,8(4):479-495.
[72] ROACH J C M,LEVETT P N,LAVOIE M C.Identification of Streptococcus iniae by commercial bacterial identification systems[J].Journal of Microbiological Methods,2006,67(1):20-26.
[73] WATTS J L.Characterization and identification of Streptococci isolated from bovine mammary glands[J].Journal of Dairy Science,1988,71(6):1616-1624.
[74] JAYARAO B M,JR J J D,BAUMBACH G A,et al.Differentiation of Streptococcus uberis from Streptococcus parauberis by polymerase chain reaction and restriction fragment length polymorphism analysis of 16S ribosomal DNA.[J].Journal of Clinical Microbiology,1991,29(12):2774-2778.
[75] BENTLEY R W,LEIGH J A,COLLINS M D.Intrageneric structure of Streptococcus based on comparative analysis of small-subunit rRNA sequences[J].International Journal of Systematic Bacteriology,1991,41(4):487-494.
[76] BENTLEY R W,LEIGH J A.Development of PCR-based hybridization protocol for identification of streptococcal species[J].Journal of Clinical Microbiology,1995,33(5):1296-1301.
[77] MISHRA A,NAM G H,GIM J A,et al.Comparative evaluation of 16S rRNA gene in world-wide strains of Streptococcus iniae and Streptococcus parauberis for early diagnostic marker[J].Genes and Genomics,2017,39(7):779-791.
[78] NGUYEN T L,LIM Y J,KIM D H,et al.Development of real-time PCR for detection and quantitation of Streptococcus parauberis[J].Journal of Fish Diseases,2016,39(1):31-39.
[79] 李亚军,耿毅,余泽辉,等.两株不同血清型鱼源无乳链球菌生物学特性的比较研究[J].中国预防兽医学报,2018,40(12):1122-1127.
LI Y J,GENG Y,YU Z H,et al,Comparative analysis of the biological characteristics of serotypes Ⅰa and Ⅱa of Streptococcus agalactiae strains from fish[J].Chinese Journal of Preventive Veterinary Medicine,2018,40(12):1122-1127.(in Chinese)
[80] 熊向英,黄国强,王志成,等.广西卵形鲳鲹海豚链球菌基因分型、耐药谱型以及毒力基因检测[J].水产学报,2018,42(4):586-595.
XIONG X Y,HUANG G Q,WANG Z C,et al.Molecular typing,antibiogram type and detection of virulence genes of Stereptococcus iniae strains isolated from golden pompano (Tranchinotus ovatus) in Guangxi Province[J].Journal of Fisheries of China,2018,42(4):586-595.(in Chinese)
[81] 包秀慧.牛乳源致病菌检测技术的发展及其对乳品安全的意义[J].中国病原生物学杂志,2020,15(7):854-858.
BAO X H.Development of technology to detect pathogens from bovine milk and its significance to the safety of dairy products[J].Journal of Parasitic Biology,2020,15(7):854-858.(in Chinese)
[82] LIU L H,YANG F,LI X P,et al.Whole-Genome sequence of Streptococcus parauberis strain SP-llh,isolated from cows with mastitis in Western China[J].Genome Announcements,2017,5(2):e01389-16.
[83] HAINES A,NEBERGALL E,BESONG E,et al.Draft genome sequences for seven Streptococcus parauberis isolates from wild fish in the Chesapeake Bay [J].Genome Announcements,2016,4(4):e00741-16.
[84] CAMERON M,SAAB M,HEIDER L,et al.Antimicrobial susceptibility patterns of environmental streptococci recovered from bovine milk samples in the Maritime Provinces of Canada[J].Frontiers in Veterinary Science,2016,3:00079.
[85] ZHANG H,YANG F,LI X P,et al.Detection of antimicrobial resistance and virulence-related genes in Streptococcus uberis and Streptococcus parauberis isolated from clinical bovine mastitis cases in northwestern China[J].Journal of Integrative Agriculture,2020,19(11):2784-2797.
[86] KACZOREK E,MALACZEWSKA J,W JCIK R,et al.Biofilm production and other virulence factors in Streptococcus spp.isolated from clinical cases of bovine mastitis in Poland[J].BMC Veterinary Research,2017,13:398.
JCIK R,et al.Biofilm production and other virulence factors in Streptococcus spp.isolated from clinical cases of bovine mastitis in Poland[J].BMC Veterinary Research,2017,13:398.
[87] MORI K,FUKUDA Y.Protective efficacy of formalin-killed serotype Ⅰ and Ⅱ vaccines for Streptococcus parauberis infection in Japanese flounder Paralichthys olivaceus[J].Fish Pathology,2012,47(3):107-110.
[88] LEE D C,KIM D H,KIM J W,et al.Efficacy of a vaccine against Streptococcus parauberis infection in starry flounder Platichthys stellatus Pallas[J].Journal of Fish Pathology,2011,24(3):189-195.
[89] JUN J W,KANG J W,GIRI S S,et al.Superiority of PLGA microparticle-encapsulated formalin-killed cell vaccine in protecting olive flounder against Streptococcus parauberis[J].Aquaculture,2019,509:67-71.
[90] HWANG J Y,KWON M G,SEO J S,et al.Current use and management of commercial fish vaccines in Korea[J].Fish and Shellfish Immunology,2020,102:20-27.
[91] KIM K I,MIN E Y,KIM T H,et al.Application of alginate microparticles incorporating formalin-inactivated Streptococcus parauberis for oral vaccination in olive flounder[J].Aquaculture International,2020,29(1):127-138.
[92] PARK S B,NHO S W,JANG H B,et al.Development of three-valent vaccine against streptococcal infections in olive flounder,Paralichthys olivaceus[J].Aquaculture,2016,461:25-31.
[93] HAN S Y,KANG B K,KANG B J,et al.Protective efficacy of a combined vaccine against Edwardsiella tarda,Streptococcus iniae and Streptococcus parauberis in farmed olive flounder,Paralichthys olivaceus[J].Fish Pathology,2011,46(4):108-111.
[94] TAKAHASHI Y,FUKUDA K,KONDO M,et al.Bacterial diseases of marine fish and development of vaccine in Japan[J].Journal of National Fisheries University,2011,60(1):51-56.
[95] 吕可,赵前程,刘婧懿,等.噬菌体在水生动物病害防治中的应用问题和解决策略[J].水产科学,2020,39(6):964-971.
LV K,ZHAO Q C,LIU J Y,et al.Application of bacteriophage in aquaculture:focusing on problems and solutions[J].Fisheries Science,2020,39(6):964-971.(in Chinese)
[96] KWON A S,KANG B J,JUN S Y,et al.Evaluating the effectiveness of Streptococcus parauberis bacteriophage STr-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture[J].Aquaculture,2017,468(1):464-471.