鱼粉鱼油适口性好、易消化吸收、必需营养素全面,是水产饲料的优质蛋白源和脂肪源。近年来,由于全球气候变化、环境污染及过度捕捞,鱼粉、鱼油产量已难以满足行业发展的需求。减少饲料中鱼粉及鱼油的使用比例,对行业的可持续发展至关重要。而植物原料来源广泛且价格低廉,是替代鱼粉、鱼油的理想原料。
研究表明,发酵菜粕替代饲料中75%的鱼粉会降低真鲷Pagrus major的生长性能及抗氧化能力[1];大豆蛋白替代金鲷Sparus aurata饲料中40%鱼粉,会对其生长及免疫能力产生负面影响,替代达到60%时会引起炎症反应[2];亚麻籽油完全替代大菱鲆Scophthalmus maximus L.饲料中的鱼油会降低血清溶菌酶活性及总抗氧化能力[3]。全部替代鱼粉鱼油会降低欧洲狼鲈Dicentrarchus labrax生长性能[4],而完全替代军曹鱼Rachycentron canadum饲料中的鱼粉鱼油,则不会影响其生长性能[5]。可见,植物原料替代鱼粉鱼油对养殖鱼的影响因鱼而异,表明不同鱼种对植物原料的适应能力有所不同,因此,有必要对此进行深入研究。
大菱鲆以其良好的肉质及较快的生长速度,在中国及欧洲被广泛养殖。本试验中,探究了混合植物蛋白及芥花油、大豆油替代鱼粉鱼油对大菱鲆幼鱼生长、体成分、生化、抗氧化及免疫指标的影响,以期为替代大菱鲆日粮中鱼粉及鱼油提供科学参考。
1 材料与方法
1.1 材料
大菱鲆幼鱼购自蓬莱宗哲养殖有限公司,养殖试验在山东省海洋资源与环境研究院循环水养殖实验室进行。
1.2 方法
1.2.1 试验饲料的配制 试验饲料设置4个组,鱼粉鱼油组(对照)以鱼粉为蛋白源,鱼油为脂肪源;鱼油组以混合植物蛋白(大豆分离蛋白、玉米蛋白粉、发酵豆粕、花生粕、谷朊粉)为蛋白源,鱼油为脂肪源;芥花油组以混合植物蛋白为蛋白源,芥花油并补充必需脂肪酸纯化油为脂肪源;大豆油组以混合植物蛋白为蛋白源,大豆油并添加混合藻粉(裂壶藻Schizochytrium sp.与拟微绿球藻Nannochloropsis sp.的比例为1.2∶1)为主要脂肪源。各组必需脂肪酸含量均满足大菱鲆幼鱼生长的需求量[6]。按照试验饲料配方,所有干物料经粉碎过0.198 mm筛后充分混匀,然后依次加入油和蒸馏水混匀后,经螺旋挤压机加工成粒径为3 mm的颗粒,60 ℃下烘干,于-20 ℃下保存待用。试验饲料配方及营养水平见表1~表5,其中,鱼粉含粗蛋白质69%、粗脂肪6.5%;大豆分离蛋白含粗蛋白质90%;玉米蛋白粉含粗蛋白质66%;发酵豆粕含粗蛋白质52%;花生粕含粗蛋白质53%;谷朊粉含粗蛋白质80%;DHA纯化油含DHA 55%;ARA纯化油含ARA 55%;裂壶藻(干物质)中含粗蛋白质9.7%、粗脂肪25.35%;拟微绿球藻(干物质)中含粗蛋白质45%、粗脂肪18.05%;诱食剂中含5’肌苷酸二钠、谷氨酸钠,二者的比例为1∶7。
1.2.2 试验设计及养殖管理 正式试验开始前,将试验鱼暂养于养殖系统中以适应养殖环境,期间投喂对照组与试验组混合的饲料。两周后逐渐减少投喂饲料中对照组饲料的比例,直至为零。10 d后,挑选健康且规格统一的大菱鲆幼鱼放入12个养殖桶(高80 cm,直径70 cm,水深50 cm)中,每桶30尾鱼(体质量为35.95 g±0.05 g),用每种饲料随机投喂3个桶,每组设3个重复,养殖试验共进行55 d。
表1 试验饲料一般营养组成(干物质)
Tab.1 Nutrients of the diets used in the experiment(dry material) w/%

组别group水分moisture粗蛋白质crude protein粗脂肪crude lipid粗灰分crude ash能量/(kJ·g-1)total energy鱼粉鱼油组FMFO4.9653.2910.4914.1020.11鱼油组FO4.7151.7910.906.5321.27芥花油组CAN4.2651.8510.276.6121.28豆油组SOY4.9251.6511.176.5921.57
表2 试验饲料配方
Tab.2 Ingredients of the diets used in the experiment %

成分composition鱼粉鱼油组FMFO 鱼油组FO 芥花油组CAN豆油组SOY鱼粉fishmeal50.000.000.000.00大豆分离蛋白soybean protein isolated0.0020.0020.0020.00玉米蛋白粉 corn gluten meal0.008.008.006.00发酵豆粕fermented soybean meal12.0022.0022.0022.00花生粕peanut meal12.0014.0014.0012.00谷朊粉gluten6.007.007.007.00α-淀粉α-starch6.006.006.006.00鱼油fish oil6.009.000.000.00豆油soybean oil0.000.000.007.00大豆卵磷脂soybean lecithin1.001.001.001.00芥花油canola oil0.000.009.000.00DHA0.000.001.200.00ARA0.000.000.100.00裂壶藻Schizochytrium sp.0.000.000.006.00拟微绿球藻Nannochloropsis sp.0.000.000.005.00诱食剂FS0.000.500.500.50牛磺酸taurine0.001.001.001.00维生素预混料vitamin premix①1.001.001.001.00矿物质预混料mineral premix②1.001.001.001.00抗氧化剂antioxident0.100.100.100.10磷酸二氢钙Ca(H2PO4)20.500.500.500.50氯化胆碱choline chloride0.500.500.500.50羧甲基纤维素钠CMC-Na3.905.334.030.48甘氨酸Gly0.001.131.131.10赖氨酸Lys0.000.900.900.84缬氨酸Val0.000.420.420.40蛋氨酸Met0.000.310.310.31精氨酸Arg0.000.310.310.27
注:①维生素预混料(mg/kg):维生素A,38;维生素D,13.2;维生素E,210;硫胺素,115;维生素K3,10;核黄素,380;盐酸吡哆醇,88;泛酸,368;烟酸,1 030;生物素,10;叶酸,20;维生素B12,1.3;肌醇,4 000;抗坏血酸,500。②矿物质预混料(mg/kg):MgSO4·7H2O,3 568;NaH2PO4·2H2O,25 568;KCl,3 020.5;KAl (SO4)2,8.3;CoCl2, 28;ZnSO4·7H2O,353;Ca-lactate,15 968;CuSO4·5H2O,9;KI,7;MnSO4·4H2O,63.1;Na2SeO3,1.5;C6H5O7Fe·5H2O,1 533;NaCl,100;NaF,4。
Note:①Vitamin premix (mg/kg diet):vitamin A,38;vitamin D,13.2;vitamin E,210;thiamin,115;vitamin K3,10;riboflavin, 380; pyridoxine HCl, 88; pantothenic acid, 368; niacin acid, 1 030; biotin, 10; folic acid, 20; vitamin B12, 1.3; inositol, 4 000; ascorbic acid, 500.②Mineral premix (mg/kg diet): MgSO4·7H2O, 3 568; NaH2PO4·2H2O, 25 568; KCl, 3 020.5; KAl (SO4)2, 8.3; CoCl2, 28; ZnSO4·7H2O, 353; Ca-lactate, 15 968; CuSO4·5H2O, 9; KI, 7; MnSO4·4H2O, 63.1; Na2SeO3, 1.5; C6H5O7Fe·5H2O, 1 533; NaCl, 100; NaF, 4.
表3 试验饲料氨基酸组成(干物质)
Tab.3 Amino acid composition of the experimental diets (dry material) %

氨基酸amino acid鱼粉鱼油组FMFO鱼油组FO芥花油组CAN豆油组SOY精氨酸 Arg3.543.293.233.30组氨酸 His1.351.321.361.26赖氨酸 Lys3.142.982.892.95蛋氨酸Met1.880.840.940.86亮氨酸Leu3.843.933.883.93异亮氨酸 Ile1.861.661.551.61苏氨酸 Thr2.262.092.092.08苯丙氨酸 Phe2.372.442.422.45缬氨酸 Val1.831.881.851.91谷氨酸 Glu9.7711.2011.2111.03天冬氨酸 Asp4.904.704.714.77丝氨酸 Ser2.512.342.372.36甘氨酸 Gly4.153.993.984.04丙氨酸 Ala3.622.832.832.85酪氨酸 Tyr1.881.761.821.82半胱氨酸 Cys0.920.960.980.97脯氨酸 Pro2.212.222.202.26总氨基酸 ∑TAA51.2450.42 50.3150.45
表4 试验饲料原料脂肪酸组成
Tab.4 Fatty acid composition of the experimental ingredients %
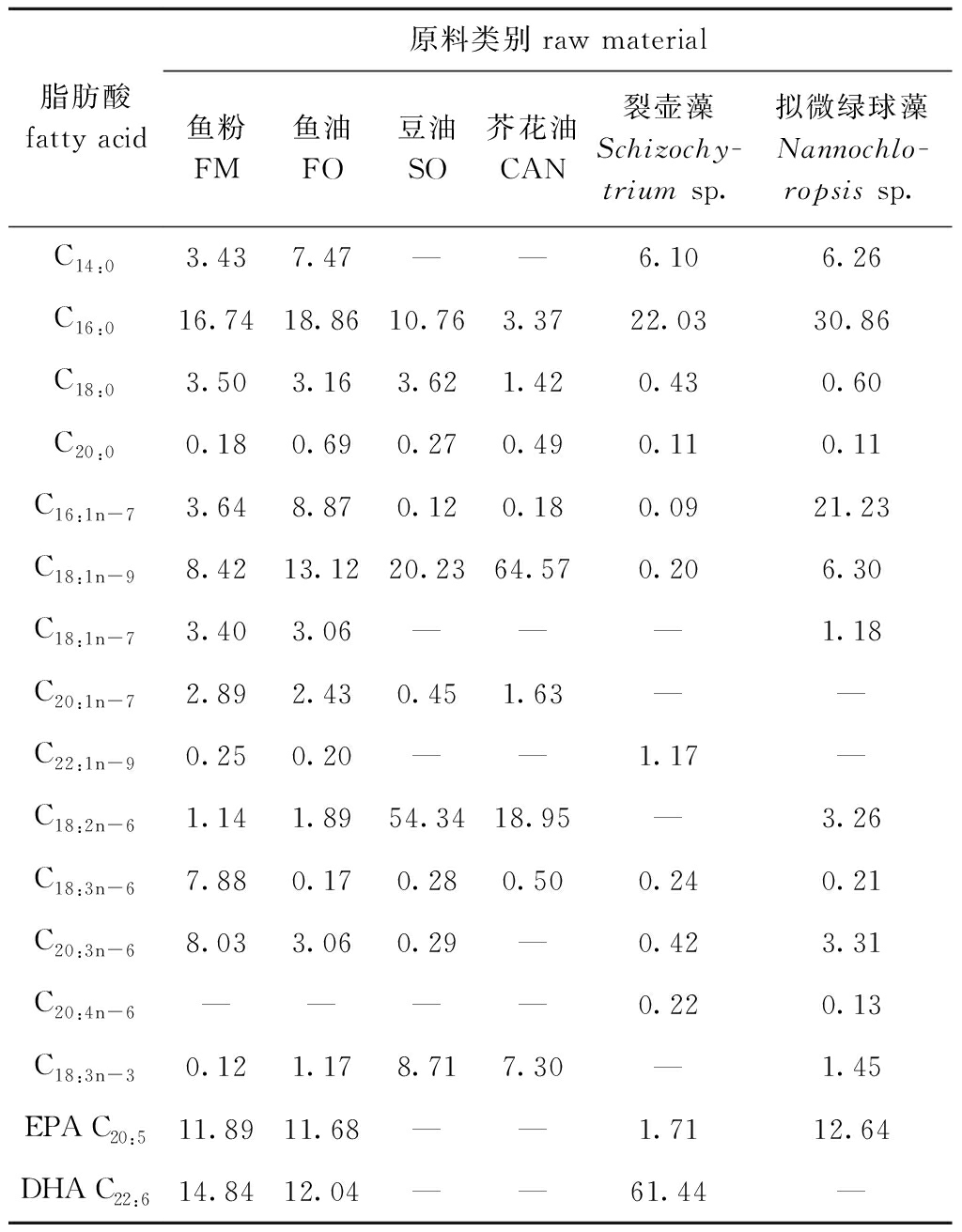
脂肪酸fatty acid原料类别raw material鱼粉FM鱼油FO豆油SO芥花油CAN裂壶藻Schizochy-trium sp.拟微绿球藻Nannochlo-ropsis sp.C14:03.437.47——6.106.26C16:016.7418.8610.763.3722.0330.86C18:03.503.163.621.420.430.60C20:00.180.690.270.490.110.11C16:1n-73.648.870.120.180.0921.23C18:1n-98.4213.1220.2364.570.206.30C18:1n-73.403.06———1.18C20:1n-72.892.430.451.63——C22:1n-90.250.20——1.17—C18:2n-61.141.8954.3418.95—3.26C18:3n-67.880.170.280.500.240.21C20:3n-68.033.060.29—0.423.31C20:4n-6————0.220.13C18:3n-30.121.178.717.30—1.45EPA C20:511.8911.68——1.7112.64DHA C22:614.8412.04——61.44—
表5 试验饲料脂肪酸组成
Tab.5 Fatty acid composition of the experimental diets %
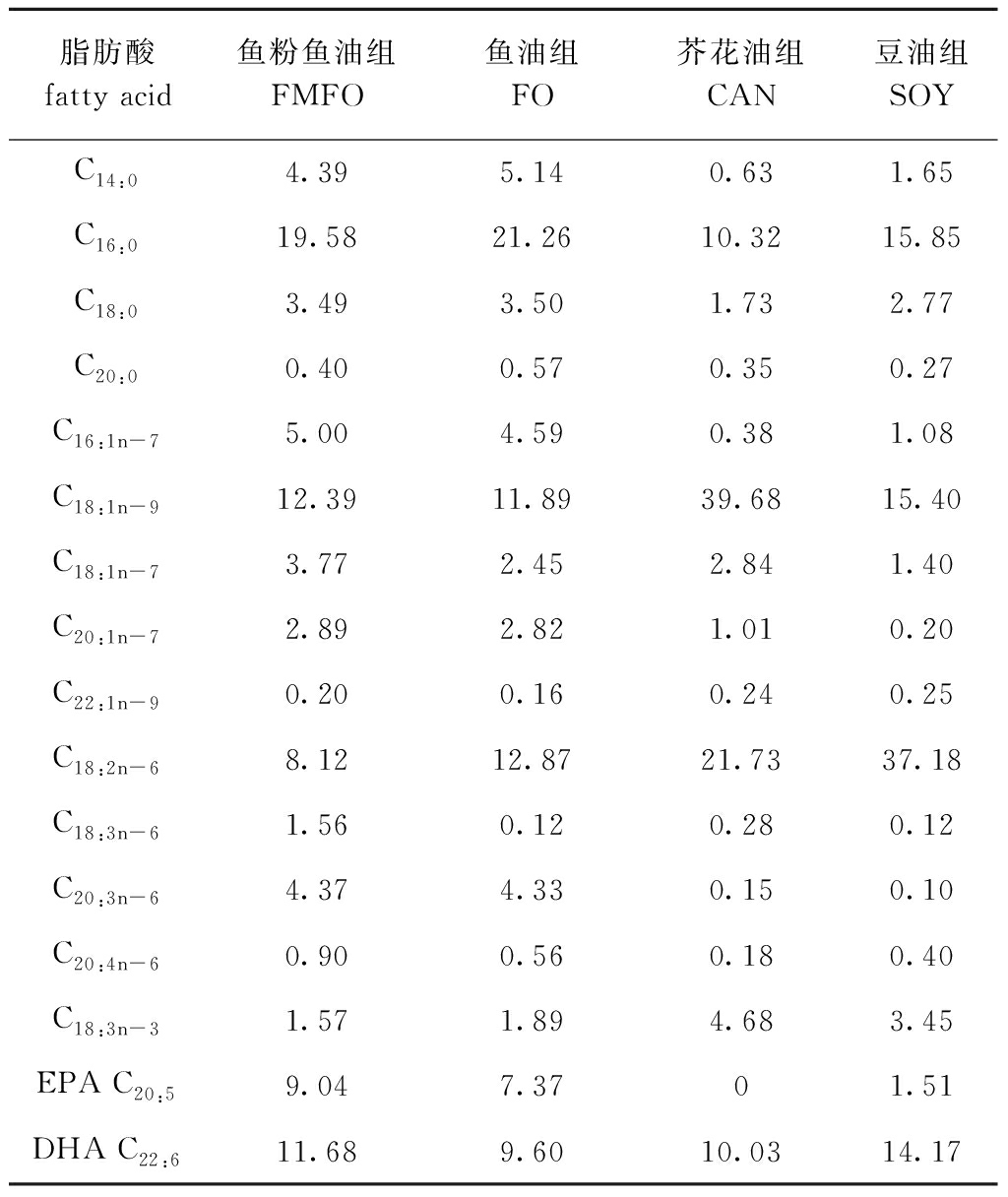
脂肪酸fatty acid鱼粉鱼油组FMFO鱼油组FO芥花油组CAN豆油组SOYC14:04.395.140.631.65C16:019.5821.2610.3215.85C18:03.493.501.732.77C20:00.400.570.350.27C16:1n-75.004.590.381.08C18:1n-912.3911.8939.6815.40C18:1n-73.772.452.841.40C20:1n-72.892.821.010.20C22:1n-90.200.160.240.25C18:2n-68.1212.8721.7337.18C18:3n-61.560.120.280.12C20:3n-64.374.330.150.10C20:4n-60.900.560.180.40C18:3n-31.571.894.683.45EPA C20:59.047.3701.51DHA C22:611.689.6010.0314.17
采取定时定量投喂(8:00和16:00),初始投喂量为鱼体质量的1%,之后根据摄食状况及时调整投喂量,投喂后30 min排残饵,记录残饵数量。养殖期间,控制水温为(17.0±0.5)℃,盐度为28~32,pH为7.8~8.2,溶解氧质量浓度>7.0 mg/L,氨氮质量浓度<0.1 mg/L。
1.2.3 样品采集 试验结束后,控食24 h,称重并计算增重率(WGR)、特定生长率(SGR)、摄食量(FI)、饲料系数(FCR)、蛋白质效率(PER)、脂肪效率(LER)及存活率(SR)。从每桶随机取8尾鱼,尾静脉取血,将血液于4 ℃冰箱静置4 h后,4 ℃下以3 500 r/min离心10 min,分离血清,分装后置于超低温冰箱(-80 ℃)中保存待测。随机取采血后的3尾鱼,冰上分离肝脏,冰浴匀浆后转移至超低温冰箱(-80 ℃)中保存,用于相关酶活测定。每桶再取3尾全鱼用于全鱼氨基酸及脂肪酸组成测定。
1.2.4 样品分析
1)饲料、全鱼、肝脏氨基酸(除色氨酸外)与脂肪酸含量测定。称取一定量的冻干样品,加入6 mL 6 mol/L HCl,80 ℃下金属浴30 min,110 ℃下金属浴24 h,赶酸后过0.22 μm有机滤膜,滤液收集于带有旋塞的样品瓶中,用氨基酸分析仪(HITACHI L-8900, 日本)分析氨基酸组成及含量。脂肪酸含量的测定步骤参考Qiao等[7]的报道,通过高效气相色谱仪(SHIMADZU GC-2010, 日本)测定。
2)血清生化指标的测定。采用南京建成生物工程研究所的试剂盒测定总蛋白质(TP)、葡萄糖(Glu)、总胆固醇(TCHO)、甘油三酯(TG)、低密度脂蛋白(LDL-C)、高密度脂蛋白(HDL-C)、酸性磷酸酶(ACP)、碱性磷酸酶(AKP)、谷丙转氨酶(ALT)及谷草转氨酶(AST)含量,具体操作方法参考试剂盒说明书。
3)血清及肝脏抗氧化相关指标测定。采用上海酶联生物科技有限公司试剂盒测定超氧化物歧化酶(SOD)和谷胱甘肽过氧化物酶(GSH-Px),采用南京建成生物工程研究所试剂盒测定丙二醛含量(MDA)、过氧化氢酶(CAT)及溶菌酶(LZM)活性,具体操作方法参照试剂盒说明书。
4)生长性能及饲料利用参数。计算公式为
WGR=(mt-m0)/m0×100%,
SGR=(lnmt/lnm0)/t×100%,
FI=F/[(m0+mt)/2×t],
FCR=F/(mt-m0),
PER=(mt-m0)/(F ×P)×100%,
LER=(mt-m0)/(F ×L)×100%,
SR=Nt/N0×100%。
其中:mt、m0分别为试验终末和试验初始鱼体平均质量(g);F为饲料摄入量(g);P、L分别为饲料粗蛋白质和粗脂肪质量分数(%);t为试验时间(d);Nt、N0分别为试验终末和初始鱼数量(ind.)。
1.3 数据处理
试验数据均以平均值±标准差(mean±S.D.)表示,采用SPSS 17.0软件对数据进行单因素方差分析(One-way ANOVA),采用Duncan法进行组间多重比较,显著性水平设为0.05。
2 结果与分析
2.1 大菱鲆生长性能及饲料利用指标的变化
从表6可见:各组试验鱼存活率无显著性差异(P>0.05);豆油组增重率、特定生长率、饲料系数、蛋白质效率与鱼粉鱼油组无显著性差异(P>0.05),二者均显著优于其他组(P<0.05),鱼油组、芥花油组各指标均无显著性差异(P>0.05);鱼粉鱼油组脂肪效率最高,豆油组次之,鱼油组及芥花油组最低(P<0.05)。
表6 植物原料替代鱼粉鱼油对大菱鲆生长性能及饲料利用的影响
Tab.6 Effects of replacing fishmeal and fish oil by plant-derived ingredients on growth performance and feed utilization of turbot juveniles

组别group终末体质量/gFBW增重率/%WGR特定生长率/(%·d-1)SGR摄食量/(g·d-1)FI饵料系数FCR蛋白质效率/%PER脂肪效率/%LER存活率/%SR鱼粉鱼油组FMFO70.46±0.53b96.02±1.64b1.25±0.02b27.97±1.89a0.83±0.03a2.29±0.05b11.63±0.27c94.44±6.94鱼油组FO64.54±0.67a79.28±1.86a1.08±0.02a31.73±0.22b1.03±0.02b1.88±0.04a8.92±0.20a97.78±1.92芥花油组CAN63.99±0.48a77.75±1.32a1.07±0.01a31.63±0.30b1.10±0.06b1.75±0.09a8.83±0.45a92.22±5.09豆油组SOY68.38±1.47b90.54±3.61b1.19±0.03b30.52±0.54ab0.88±0.04a2.20±0.10b10.16±0.46b100.00±0.00
注:同列中标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05),下同。
Note:The means with different letters within the same column are significantly different in the groups at the 0.05 probability level, and the means with the same letter within the same column are not significant differences, et sequentia.
2.2 大菱鲆体成分的变化
从表7可见:各组粗蛋白质及水分含量无显著性差异(P<0.05);鱼粉鱼油组与豆油组粗脂肪含量无显著性差异(P>0.05),但均显著高于其他组(P<0.05);鱼粉鱼油组粗灰分含量最高,鱼油组、芥花油组次之,豆油组最低(P<0.05)。
表7 植物原料替代鱼粉鱼油对大菱鲆体组成成分的影响(湿物质)
Tab.7 Effects of replacing fishmeal and fish oil by plant-derived ingredients on proximate body composition of turbot juveniles (wet material) w/%

组别group水分moisture粗蛋白质crude protein粗脂肪crude lipid粗灰分crude ash鱼粉鱼油组 FMFO76.35±0.4017.41±0.131.77±0.09b4.50±0.03c鱼油组 FO76.76±0.4717.51±0.031.52±0.03a4.30±0.02b芥花油组CAN76.85±0.5417.53±0.031.53±0.04a4.28±0.02b豆油组 SOY76.79±0.3817.50±0.211.93±0.13b3.74±0.04a
2.3 大菱鲆全鱼及肝脏氨基酸、脂肪酸组成的变化
从表8可见:大菱鲆全鱼氨基酸组成中,氨基酸总量及必需氨基酸总量显著高于鱼粉鱼油组(P<0.05);除Met及Phe外,其余各种必需氨基酸均有不同程度的提高,豆油组Met含量最高,各组Phe含量无显著性差异(P>0.05);各组∑NEAA无显著性差异(P>0.05),鱼油组、芥花油组、豆油组Gly、Pro、Ala含量总体上显著低于鱼粉鱼油组(P<0.05)。
表8 植物原料替代鱼粉鱼油对大菱鲆全鱼氨基酸组成的影响(干物质)
Tab.8 Effects of replacing fishmeal and fish oil by plant-derived ingredients on whole body amino acid composition of turbot juveniles (dry material) %
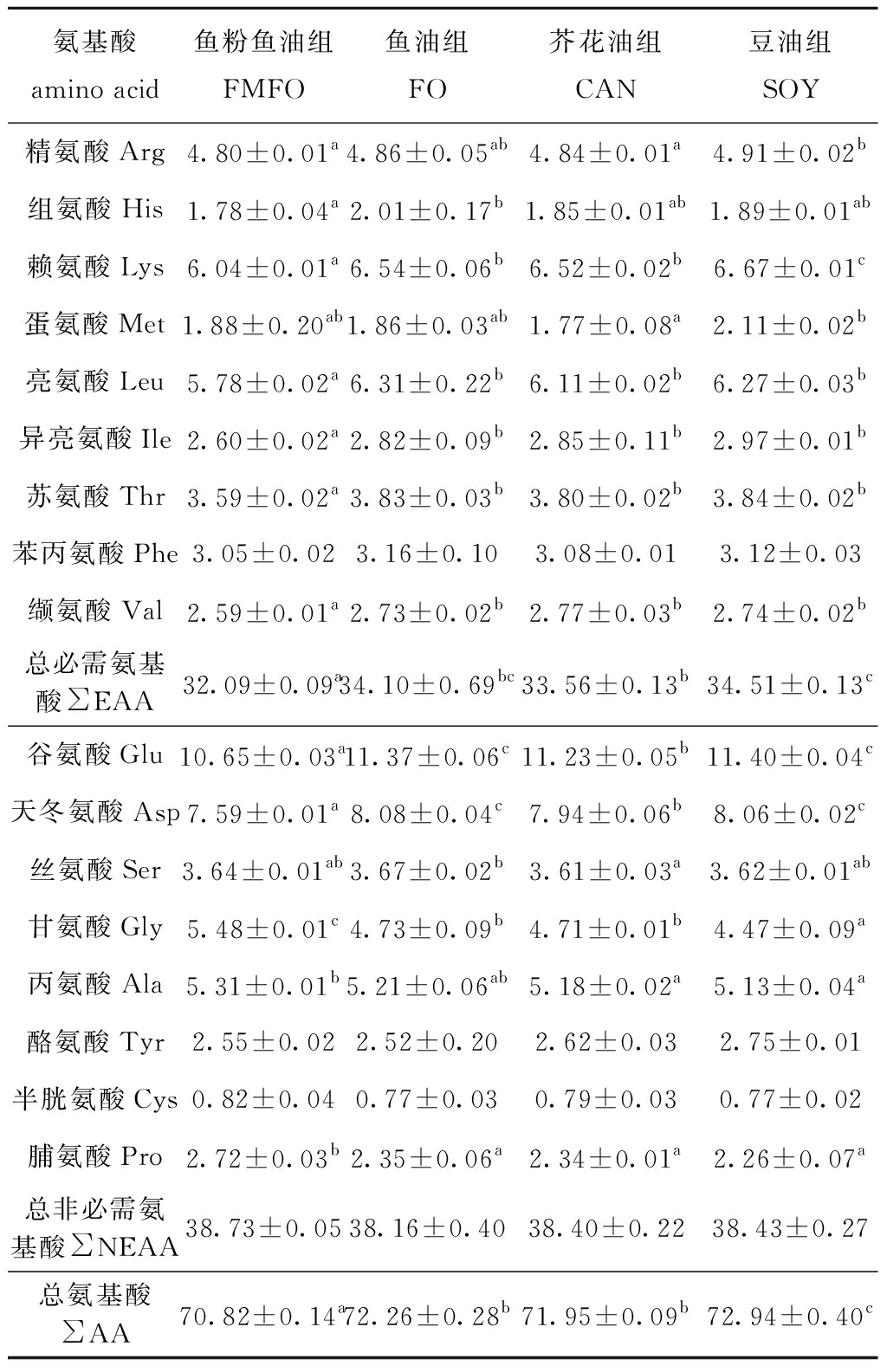
氨基酸 amino acid鱼粉鱼油组FMFO鱼油组FO芥花油组CAN豆油组SOY精氨酸Arg4.80±0.01a4.86±0.05ab4.84±0.01a4.91±0.02b组氨酸His1.78±0.04a2.01±0.17b1.85±0.01ab1.89±0.01ab赖氨酸Lys6.04±0.01a6.54±0.06b6.52±0.02b6.67±0.01c蛋氨酸Met1.88±0.20ab1.86±0.03ab1.77±0.08a2.11±0.02b亮氨酸Leu5.78±0.02a6.31±0.22b6.11±0.02b6.27±0.03b异亮氨酸Ile2.60±0.02a2.82±0.09b2.85±0.11b2.97±0.01b苏氨酸Thr3.59±0.02a3.83±0.03b3.80±0.02b3.84±0.02b苯丙氨酸Phe3.05±0.023.16±0.103.08±0.013.12±0.03缬氨酸Val2.59±0.01a2.73±0.02b2.77±0.03b2.74±0.02b总必需氨基酸∑EAA32.09±0.09a34.10±0.69bc33.56±0.13b34.51±0.13c谷氨酸Glu10.65±0.03a11.37±0.06c11.23±0.05b11.40±0.04c天冬氨酸Asp7.59±0.01a8.08±0.04c7.94±0.06b8.06±0.02c丝氨酸Ser3.64±0.01ab3.67±0.02b3.61±0.03a3.62±0.01ab甘氨酸Gly5.48±0.01c4.73±0.09b4.71±0.01b4.47±0.09a丙氨酸Ala5.31±0.01b5.21±0.06ab5.18±0.02a5.13±0.04a酪氨酸Tyr2.55±0.022.52±0.202.62±0.032.75±0.01半胱氨酸Cys0.82±0.040.77±0.030.79±0.030.77±0.02脯氨酸Pro2.72±0.03b2.35±0.06a2.34±0.01a2.26±0.07a总非必需氨基酸∑NEAA38.73±0.0538.16±0.4038.40±0.2238.43±0.27总氨基酸∑AA70.82±0.14a72.26±0.28b71.95±0.09b72.94±0.40c
注:同行中标有不同字母者表示组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05),下同。
Note:The means with different letters within the same line are significantly different in the groups at the 0.05 probability level, and the means with the same letter within the same line are not significant differences, et sequentia.
从表9可见:大菱鲆肝脏氨基酸组成中,芥花油组及豆油组Met含量较鱼粉鱼油组显著下降(P<0.05),鱼油组、芥花油组及豆油组其余必需氨基酸较鱼粉鱼油组有一定程度的提高;对于非必需氨基酸,豆油组Ala含量显著低于其他组(P<0.05),鱼油组、芥花油组及豆油组其余非必需氨基酸较鱼粉鱼油组有一定程度提高。
表9 植物原料替代鱼粉鱼油对大菱鲆肝脏氨基酸组成的影响(干物质)
Tab.9 Effects of replacing fishmeal and fish oil by plant-derived ingredients on liver amino acid composition of turbot juveniles (dry material) %
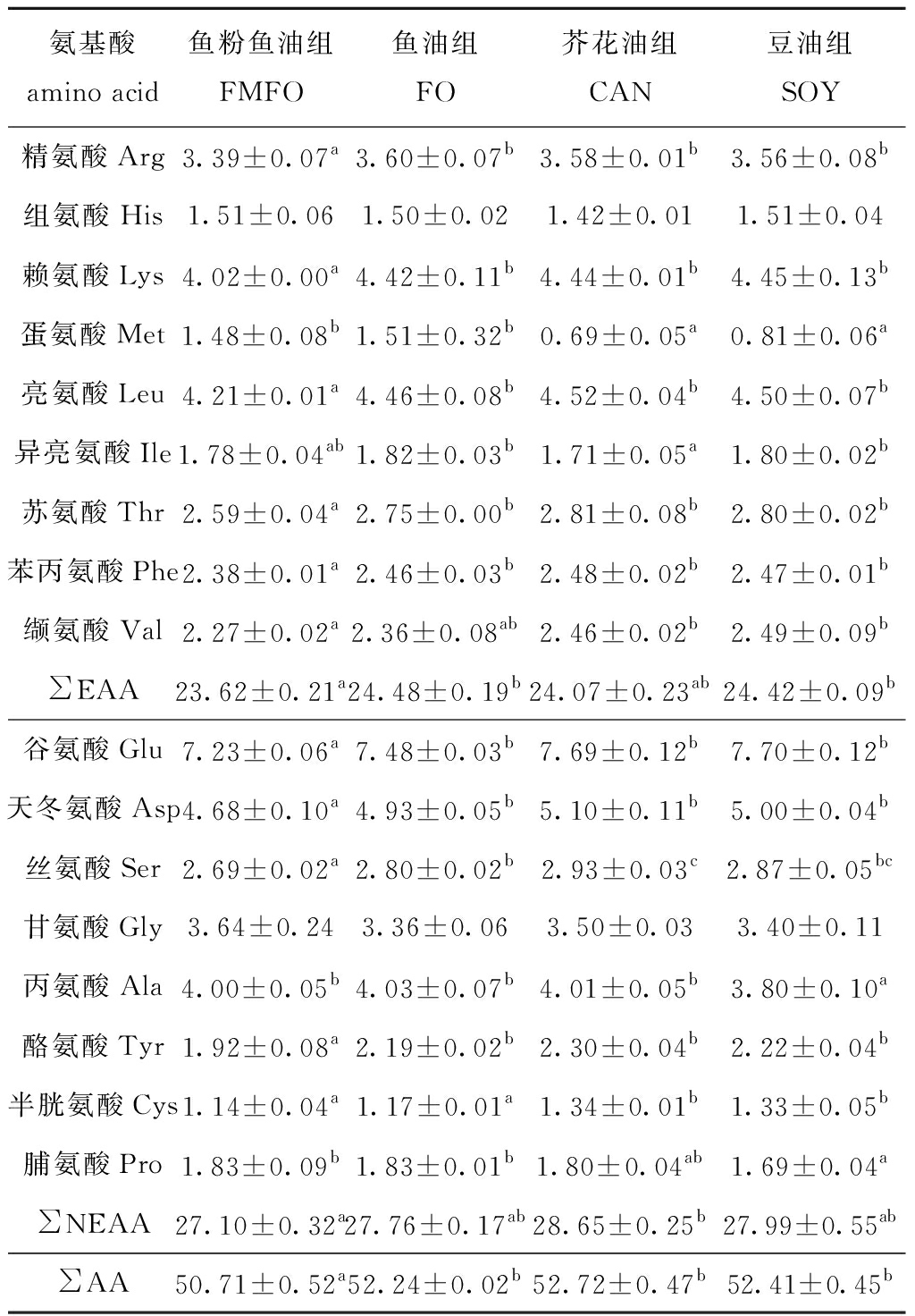
氨基酸amino acid鱼粉鱼油组FMFO 鱼油组FO芥花油组CAN豆油组SOY精氨酸Arg3.39±0.07a3.60±0.07b3.58±0.01b3.56±0.08b组氨酸His1.51±0.061.50±0.021.42±0.011.51±0.04赖氨酸Lys4.02±0.00a4.42±0.11b4.44±0.01b4.45±0.13b蛋氨酸Met1.48±0.08b1.51±0.32b0.69±0.05a0.81±0.06a亮氨酸Leu4.21±0.01a4.46±0.08b4.52±0.04b4.50±0.07b异亮氨酸Ile1.78±0.04ab1.82±0.03b1.71±0.05a1.80±0.02b苏氨酸Thr2.59±0.04a2.75±0.00b2.81±0.08b2.80±0.02b苯丙氨酸Phe2.38±0.01a2.46±0.03b2.48±0.02b2.47±0.01b缬氨酸Val2.27±0.02a2.36±0.08ab2.46±0.02b2.49±0.09b∑EAA23.62±0.21a24.48±0.19b24.07±0.23ab24.42±0.09b谷氨酸Glu7.23±0.06a7.48±0.03b7.69±0.12b7.70±0.12b天冬氨酸Asp4.68±0.10a4.93±0.05b5.10±0.11b5.00±0.04b丝氨酸Ser2.69±0.02a2.80±0.02b2.93±0.03c2.87±0.05bc甘氨酸Gly3.64±0.243.36±0.063.50±0.033.40±0.11丙氨酸Ala4.00±0.05b4.03±0.07b4.01±0.05b3.80±0.10a酪氨酸Tyr1.92±0.08a2.19±0.02b2.30±0.04b2.22±0.04b半胱氨酸Cys1.14±0.04a1.17±0.01a1.34±0.01b1.33±0.05b脯氨酸Pro1.83±0.09b1.83±0.01b1.80±0.04ab1.69±0.04a∑NEAA27.10±0.32a27.76±0.17ab28.65±0.25b27.99±0.55ab∑AA50.71±0.52a52.24±0.02b52.72±0.47b52.41±0.45b
从表10可见:大菱鲆全鱼脂肪酸组成中,鱼粉鱼油组∑SFA显著高于其他组(P<0.05),鱼油组次之,芥花油组最低;芥花油组C18:1n-9、∑MUFA显著高于其他组(P<0.05);鱼油组、芥花油组及豆油组∑n-6PUFA显著高于鱼粉鱼油组(P<0.05),豆油组C18:2n-6显著高于其他组(P<0.05);鱼粉鱼油组、鱼油组∑n-3PUFA、EPA含量显著高于其他组(P<0.05),而芥花油组最低,鱼粉鱼油组DHA含量显著高于其他组(P<0.05),而鱼油组最低。
表10 植物原料替代鱼粉鱼油对大菱鲆全鱼脂肪酸组成的影响
Tab.10 Effects of replacing fishmeal and fish oil by plant-derived ingredients on whole body fatty acid profile of turbot juveniles %

脂肪酸 fatty acid鱼粉鱼油组FMFO鱼油组FO芥花油组CAN豆油组SOYC14:03.38±0.01c3.75±0.10d1.06±0.07a1.89±0.01bC16:018.35±0.23c17.21±0.15b12.66±0.69a16.84±0.03bC18:04.47±0.18c3.91±0.13b3.29±0.18a4.50±0.03cC20:00.30±0.02c0.29±0.03bc0.27±0.01abc0.24±0.00a∑SFA26.49±0.03c25.15±0.15d17.00±0.46a23.46±0.06bC16:1n-74.11±0.09c4.42±0.09d0.75±0.05a1.59±0.01bC18:1n-913.99±0.22a14.37±0.05a30.15±0.99b14.51±0.15aC18:1n-73.55±0.05c2.84±0.12b2.88±0.01b1.89±0.00aC20:1n-72.30±0.03c2.15±0.15c0.99±0.06a0.81±0.01aC22:1n-90.26±0.03a0.23±0.00a0.54±0.05c0.33±0.00b∑MUFA24.25±0.25b23.92±0.07b35.60±0.51c19.13±0.17aC18:2n-69.50±0.53a14.49±0.37b19.56±0.23c28.36±0.05dC18:3n-60.15±0.02ab0.12±0.02a0.18±0.02b0.13±0.00aC20:3n-61.98±0.05c1.63±0.20b0.20±0.04a0.42±0.01aC20:4n-61.81±0.30c1.34±0.02b0.87±0.03a1.28±0.03b∑n-6PUFA13.43±0.17a17.35±0.20b20.82±0.19c30.19±0.03dC18:3n-30.98±0.06a1.46±0.02b2.55±0.17c1.62±0.03bEPA C20:55.74±0.05c6.55±0.18d0.76±0.03a1.58±0.02bDHA C22:617.38±0.41c15.87±0.16a16.52±0.21b16.95±0.09bc∑n-3PUFA23.98±0.16b23.87±0.32b19.83±0.11a20.15±0.09a
从表11可见:大菱鲆肝脏脂肪酸组成中,芥花油组及豆油组∑SFA显著降低(P<0.05);芥花油组C18:1n-9及∑MUFA显著高于其他组(P<0.05),而豆油组∑MUFA则最低(P<0.05);芥花油组及豆油组C18:2n-6及∑n-6PUFA显著高于其他组(P<0.05);芥花油组及豆油组EPA、DHA及∑n-3PUFA显著低于其他组(P<0.05)。
表11 植物原料替代鱼粉鱼油对大菱鲆肝脏脂肪酸组成的影响
Tab.11 Effects of replacing fishmeal and fish oil by plant-derived ingredients on liver fatty acid profile of turbot juveniles %

脂肪酸 fatty acid鱼粉鱼油组FMFO鱼油组FO芥花油组CAN豆油组SOYC14:04.97±0.00c6.00±0.02d1.25±0.02a2.04±0.01bC16:018.22±0.15c18.13±0.04c11.25±0.10a13.66±0.03bC18:02.42±0.01d2.34±0.02c1.02±0.03a1.36±0.01bC20:00.16±0.00d0.12±0.00c0.07±0.00a0.08±0.00b∑SFA25.77±0.16c26.59±0.04d13.59±0.15a17.15±0.03bC16:1n-76.74±0.09c6.10±0.02b1.10±0.06a1.04±0.04aC18:1n-913.04±0.12a13.54±0.09b39.88±0.08d17.35±0.13cC18:1n-74.52±0.03c3.22±0.02b0.01±0.00a0.01±0.00aC20:1n-72.09±0.01d1.62±0.13c0.72±0.02b0.25±0.02aC22:1n-90.28±0.00b0.23±0.00a0.43±0.00d0.37±0.00c∑MUFA26.66±0.24c24.72±0.22b42.13±0.02d19.03±0.18aC18:2n-69.22±0.02a18.33±0.25b27.25±0.15c47.85±0.22dC18:3n-60.47±0.370.08±0.000.28±0.000.13±0.00C20:3n-61.36±0.02d0.83±0.01c0.07±0.01a0.11±0.00bC20:4n-61.61±0.06d1.24±0.01c0.29±0.03a0.48±0.02b∑n-6PUFA12.67±0.39a20.49±0.23b27.89±0.11c48.57±0.20dC18:3n-31.24±0.01a1.93±0.01b5.26±0.03d4.01±0.01cEPA C20:56.67±0.00d5.64±0.02c0.44±0.02a0.58±0.01bDHA C22:614.30±0.29d10.30±0.02c5.95±0.04a7.31±0.03b∑n-3PUFA22.20±0.28c17.86±0.05b11.65±0.04a11.89±0.08a
2.4 大菱鲆幼鱼血清生化指标的变化
从表12可见:各组血清Glu、TP及T-CHO含量均无显著性差异(P>0.05);各替代组TG含量显著高于鱼粉鱼油组(P<0.05);芥花油组LDL-C含量显著高于鱼油组、鱼粉鱼油组(P<0.05);鱼粉鱼油组、鱼油组HDL-C含量显著高于其他组(P<0.05);各组间ACP、AST活性无显著性差异(P>0.05),芥花油组及豆油组AKP和ALT活性显著高于鱼粉鱼油组(P<0.05)。
表12 植物原料替代鱼粉鱼油对大菱鲆幼鱼血清生化指标的影响
Tab.12 Effects of replacing fishmeal and fish oil by plant-derived ingredients on serum biochemical indices of turbot juveniles
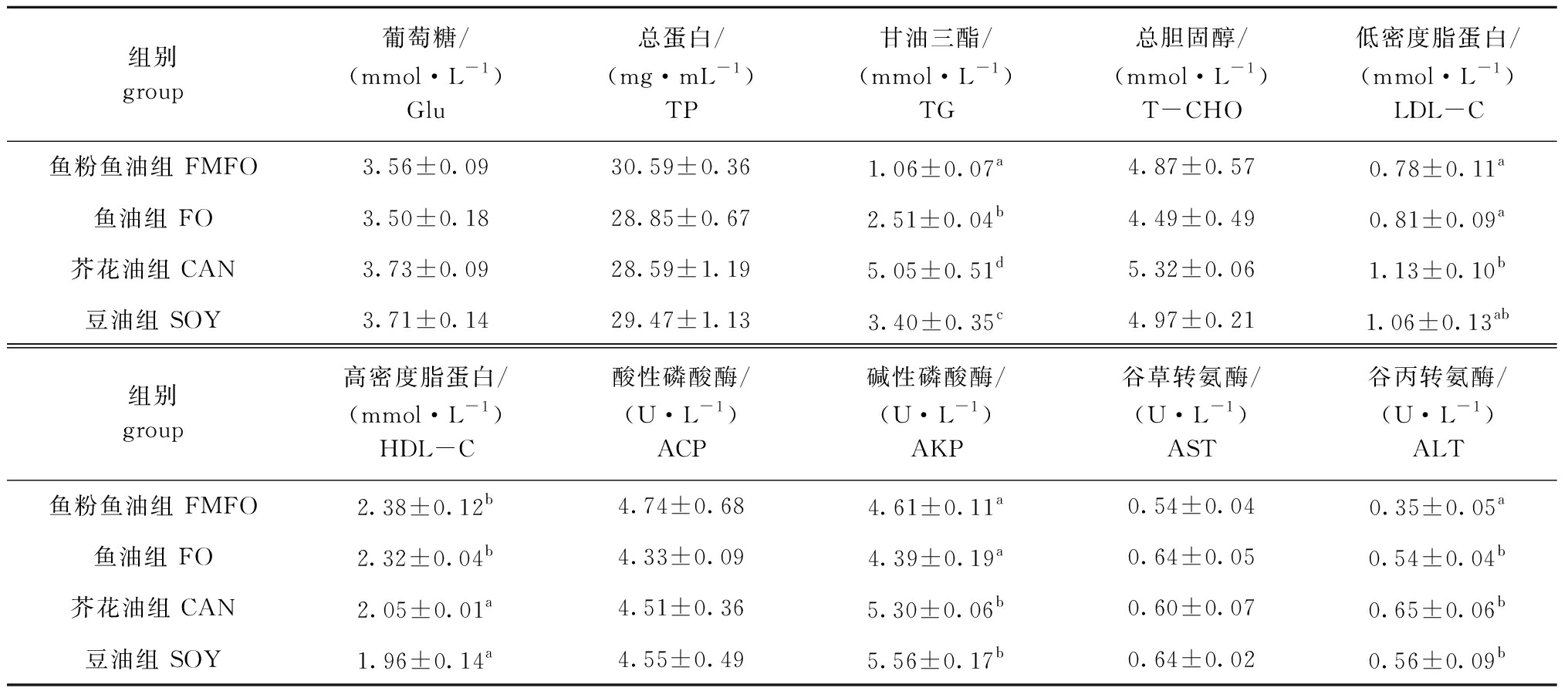
组别group葡萄糖/(mmol·L-1)Glu总蛋白/(mg·mL-1)TP甘油三酯/(mmol·L-1)TG总胆固醇/(mmol·L-1)T-CHO低密度脂蛋白/(mmol·L-1)LDL-C鱼粉鱼油组 FMFO3.56±0.0930.59±0.361.06±0.07a4.87±0.570.78±0.11a鱼油组 FO3.50±0.1828.85±0.672.51±0.04b4.49±0.490.81±0.09a芥花油组 CAN3.73±0.0928.59±1.195.05±0.51d5.32±0.061.13±0.10b豆油组 SOY3.71±0.1429.47±1.133.40±0.35c4.97±0.211.06±0.13ab组别group高密度脂蛋白/(mmol·L-1)HDL-C酸性磷酸酶/(U·L-1)ACP碱性磷酸酶/(U·L-1)AKP谷草转氨酶/(U·L-1)AST谷丙转氨酶/(U·L-1)ALT鱼粉鱼油组 FMFO2.38±0.12b4.74±0.684.61±0.11a0.54±0.040.35±0.05a鱼油组 FO2.32±0.04b4.33±0.094.39±0.19a0.64±0.050.54±0.04b芥花油组 CAN2.05±0.01a4.51±0.365.30±0.06b0.60±0.070.65±0.06b豆油组 SOY1.96±0.14a4.55±0.495.56±0.17b0.64±0.020.56±0.09b
2.5 大菱鲆幼鱼血清与肝脏抗氧化能力的变化
从表13可见:各组血清中SOD、CAT、GSH-Px活性及MDA含量无显著性差异(P>0.05),芥花油组、豆油组血清中LZM活性显著低于其他组(P<0.05);豆油组肝脏SOD活性最低,但与其他3组间无显著性差异(P>0.05);芥花油组MDA含量显著高于鱼粉鱼油组和鱼油组(P<0.05);鱼粉鱼油组的CAT和LZM活性显著高于其他组(P<0.05);鱼油组、芥花油组GSH-Px活性显著低于鱼粉鱼油组(P<0.05)。
表13 植物原料替代鱼粉鱼油对大菱鲆幼鱼血清与肝脏抗氧化酶活力的影响
Tab.13 Effects of replacing fishmeal and fish oil by plant-derived ingredients on antioxidant enzyme activities in serum and liver of turbot juveniles

组别group血清serum超氧化物歧化酶/(U·mL-1)SOD丙二醛/(nmol·mL-1)MDA过氧化氢酶/(U·mL-1)CAT谷胱甘肽过氧化物酶/(U·L-1)GSH-Px溶菌酶/(U·L-1)LZM鱼粉鱼油组FMFO164.67±5.829.62±0.762.52±0.41113.42±6.0610.07±0.26b鱼油组FO167.67±6.699.25±0.232.20±0.17116.95±14.3710.05±0.27b芥花油组CAN164.93±5.579.50±1.022.17±0.00122.84±8.648.32±10.07a豆油组SOY157.76±1.2910.19±0.622.39±0.01113.52±14.338.39±10.06a组别group肝脏liver超氧化物歧化酶/(U·mg-1prot)SOD丙二醛/(nmol·mg-1prot)MDA过氧化氢酶/(U·mg-1prot)CAT谷胱甘肽过氧化物酶/(U·mg-1prot)GSH-Px溶菌酶/(U·mg-1prot)LZM鱼粉鱼油组FMFO1500.39±47.800.41±0.01a2.35±0.09b1207.63±23.87b73.02±1.22b鱼油组FO1490.37±50.990.42±0.00a1.60±0.06a1098.60±22.29a64.25±3.59a芥花油组CAN1436.44±39.470.47±0.01b1.57±0.09a1063.55±12.36a65.29±3.11a豆油组SOY1413.18±56.390.44±0.02ab1.59±0.04a1132.87±49.58ab63.22±4.75a
3 讨论
3.1 植物原料替代鱼粉鱼油对大菱鲆生长性能及饲料利用的影响
本试验中,经过8周的养殖试验,鱼油组、芥花油组试验鱼生长性能与饲料利用率均显著低于鱼粉鱼油组、豆油组,而豆油组与鱼粉鱼油组增重率、特定生长率、摄食量、饵料系数及蛋白效率无显著性差异。鱼粉不仅营养全面,而且是天然的诱食剂。研究者对虹鳟Oncorhynchus mykiss[8]、黄尾魳Seriola quinqueradiata[9]及金鲷[10]等的试验发现,低增重率主要是由鱼粉替代后摄食量降低导致。本试验中,鱼油组、芥花油组及豆油组摄食量显著高于鱼粉鱼油组,可能与添加的诱食剂有关。充足的摄食是生长的必然前提,然而并不一定带来良好的生长表现,如本试验中鱼油组、芥花油组摄食量虽高于鱼粉鱼油组、豆油组,但饵料系数却显著高于鱼粉鱼油组、豆油组,即饲料效率(feed efficiency, FE)低于鱼粉鱼油组、豆油组,故导致鱼油组、芥花油组增重率低于鱼粉鱼油组、豆油组。这一结果与对日本真鲈Lateolabrax japonicus的研究结果一致[11],以大豆蛋白替代75%的鱼粉时,该组有较高的摄食量,但饲料效率较低,故增重率亦较低。
饲料由蛋白质及脂肪两大主要成分组成,蛋白质效率、脂肪效率和饲料效率本质均反映试验动物对饲料的利用情况。本试验中,由于鱼粉鱼油组添加了较其他组精细植物蛋白原料粗糙的鱼粉,而豆油组添加了颗粒蓬松的裂壶藻粉,因此,这两组饲料颗粒较鱼油组及芥花油组蓬松,而鱼油组、芥花油组饲料添加的精细植物蛋白料颗粒较硬,推测这可能会影响试验鱼的摄食及消化吸收,进而影响各组的增重率、饵料系数、蛋白质效率和脂肪效率。Andrew等[12] 对金鲷的研究表明,相较于普通饲料,质地柔软的饲料更有利于消化吸收。Aas等[13] 发现,经海水浸泡的饲料相较于干料能够更快地从大西洋鲑的胃中转运到小肠,更利于消化与吸收。由于饲料质地对试验鱼消化吸收的相关报道较少,因此,在这方面仍需进一步的研究。此外,本试验中虽使用多种植物蛋白源,并补充相应的晶体氨基酸,但由于鱼体对晶体氨基酸与饲料蛋白质中结合态氨基酸在消化吸收时间上的不同步,会造成氨基酸利用过程中的不平衡,另外,饲料在制作过程中,晶体氨基酸的损失也会影响氨基酸的平衡。Gao等[14]研究发现,蛋氨酸缺乏会降低大菱鲆的生长及饲料利用效果,且会对肠道有一定损伤。Peres等[15]研究表明,赖氨酸含量与大菱鲆的生长密切相关。因此,氨基酸的不平衡也是导致各处理组生长性能低于鱼粉鱼油组的原因。
3.2 植物原料替代鱼粉鱼油对大菱鲆营养组成及氨基酸、脂肪酸组成的影响
本试验中,各组全鱼水分含量及粗蛋白质含量无显著性差异,说明植物原料替代鱼粉鱼油并未对全鱼粗蛋白质及水分沉积造成影响。鱼粉鱼油组、豆油组全鱼粗脂肪含量显著高于鱼油组、芥花油组,主要是由于鱼油组及芥花油组脂肪效率较低导致,故此两组鱼脂肪沉积较少。此外,鱼油组、芥花油组饲料效率低,可利用的营养物质少,在某种意义上鱼处于饥饿状态,养殖鱼可能需要消耗更多脂肪以提供机体生命活动的能量,故而减少了机体的脂肪含量。这与Yang等[16]对牙鲆Paralichthys olivaceus及Li等[17]对异育银鲫Carassius auratus gibelio var.CAS III的研究结果类似,本研究结果表明,饥饿会促进脂肪分解产能。
相较于鱼粉鱼油组,鱼油组、芥花油组及豆油组全鱼氨基酸组成中的各必需氨基酸含量均有不同程度的提高,而与全鱼氨基酸不同的是,芥花油组及豆油组肝脏中Met含量则显著降低。本试验中,虽采用多种植物蛋白添加相应的晶体氨基酸替代鱼粉,使得氨基酸模式与对照组接近,但机体内晶体氨基酸与蛋白质消化吸收不同步[18-19],未同步消化的氨基酸进入鱼机体主要代谢器官,而后继续利用,重新分配,以保证机体正常生长。肝脏是机体主要的营养代谢器官,且是调节代谢的中枢器官,因此,推测肝脏氨基酸中Met显著下降的原因有两方面,一是饲料中缺乏Met;二是由于饲料中缺乏Met,而机体又无法合成Met,肝脏通过调节代谢,减少肝脏中Met的沉积,使Met更多的流向机体,以保证机体的正常生长,从而对全鱼氨基酸组成影响较小。根据Skiba-Cassy等[20]对虹鳟的研究,投喂缺乏Met的饲料,会提高与氨基酸代谢相关基因的表达量,激活可以减少蛋白质合成、促进氨基酸合成及转运的GCN2/eIF2α(GCN2,general control non-derepressible 2; eIF2α,elongation initiation factor 2α)通路。而根据Panserat等[21]对虹鳟的研究,投喂100%植物源饲料时虹鳟肝脏57%的差异表达基因的功能与代谢相关,而其中37%和蛋白质代谢紧密相关。氨基酸不平衡会影响肝脏氨基酸组成,同样也影响到鱼的生长性能,具体表现在饲喂不同饲料的鱼,若鱼达到相同体质量,氨基酸不平衡饲料组所用时间较长,相同时间下,增重率及饲料效率均较低。
植物油缺乏鱼油富含的多不饱和脂肪酸(PUFA),尤其是海水鱼生长所必需的DHA及EPA[22]。裂壶藻富含DHA[23],在鱼油替代的试验中常作为DHA的重要来源[24]。拟微绿球藻富含EPA,常作为轮虫卤虫的EPA供给源[25],也作为EPA来源应用在日本对虾Marsupenaeus japonicus[26]、牙鲆[27]幼体的营养强化中。本试验结果表明,全鱼及肝脏脂肪酸组成反映了各组饲料的脂肪酸组成特点。这与利用植物油替代大菱鲆[28]、金鲷[29]和军曹鱼[30]饲料中鱼油的研究结果一致。长链多不饱和脂肪酸(long-chain polyunsaturated fatty acids,LC-PUFA)的合成是一个以18个碳原子的脂肪酸长链(亚油酸C18:2n-6、亚麻酸C18:3n-3)为底物,通过Δ6脂肪酸去饱和酶(Δ6 fatty acyl desaturase, Fad)、延长酶及Δ5去饱和酶(Δ5 Fad)进行合成的过程。 本试验中,替代组全鱼脂肪酸中C20:4n-6、EPA及DHA的减少,反映了大菱鲆中C18:2n-6转化成C20:4n-6,C18:3n-3转化成EPA、DHA的能力有限。
3.3 植物原料替代鱼粉鱼油对大菱鲆幼鱼血清生化指标的影响
本试验中,鱼油组、芥花油组及豆油组甘油三酯含量显著高于鱼粉鱼油组,这与Li等[31]利用小麦胚芽油替代珍珠龙胆石斑鱼Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂日粮中鱼油的试验结果类似。LDL主要转运来自肝脏的内源性胆固醇,并通过血液送至各个组织和器官,HDL的功能则与其相反,主要转运外源性胆固醇至肝脏。本试验中,鱼粉鱼油组、鱼油组及豆油组LDL-C低于芥花油组,而HDL-C含量则与之相反,这与Li等[32]对金鲳的研究结果一致。该试验结果表明,随着饲料中n-3HUFA的升高,血清中TG、T-CHO及LDL-C含量显著下降。由此可见,饲料中适宜的n-3PUFA含量能促进脂肪的代谢,这也是导致本试验中脂肪效率差异显著的主要原因。
血清中Glu含量可作为鱼类应激反应的指示指标,而血清中TP含量也可一定程度上反映鱼类非特异性免疫能力。本试验中,各组血清Glu、TP含量无显著性差异,这与Glencross等[33]对尖吻鲈上的研究结果一致。表明植物原料替代鱼粉鱼油对大菱鲆血清中Glu及TP的代谢未产生负面影响。
肝脏AST和ALT是反映肝脏健康状态的重要指示酶,当肝脏受到外界毒素胁迫或代谢出现紊乱致使肝功能受损时,肝脏细胞被破坏,肝脏中转氨酶进入血液[34]。本试验中各组血清AST水平无显著性差异,但鱼油组、芥花油组及豆油组ALT水平则显著高于鱼粉鱼油组。Li等[35]利用大豆浓缩蛋白替代星斑川鲽Platichthys stellatus饲料中的鱼粉,随着替代水平的升高,血清中AST及ALT水平升高。棉籽粕在低水平替代鱼粉(最高替代水平为7%)的情况下,不会提高武昌鱼Megalobrama amblycephala血清中两种转氨酶的水平[36],对肝脏损伤较小,但高水平(最高替代水平为60%)替代下,则会显著提高乌苏里鲇Pseudobagrus ussuriensis血清中转氨酶水平,对肝脏造成损伤[37]。而Song等[38]利用大豆水解蛋白替代星斑川鲽饲料中鱼粉时发现,即便替代100%的鱼粉,其血清中两种转氨酶的水平与对照组也无显著差异。Dossou等[1]用发酵菜籽粕替代真鲷日粮中的鱼粉时也发现,在所有替代水平下,血清中两种转氨酶水平与对照组均无显著性差异。综上,利用植物蛋白替代鱼粉可能会导致血清中AST及ALT水平升高,并造成肝脏损伤,而植物原料中抗营养因子是导致肝脏损伤的主要原因[39],同时,植物原料替代鱼粉时不同替代水平或饲喂不同养殖对象,对肝脏的影响是不同的。经发酵或者水解后的蛋白源,抗营养因子被有效降低或剔除,其对肝脏损伤程度明显降低或无损伤。另外,有研究表明,植物油替代鱼油同样会对肝脏造成损伤,Wang等[3]用亚麻籽油替代大菱鲆日粮中鱼油的试验发现,随着替代水平升高,血清中ALT水平也显著升高,这可能是由于植物油缺乏高不饱和脂肪酸所导致的。本试验中同时替代鱼粉及鱼油,与鱼粉鱼油组相比,其余3组在血清ALT水平均有所升高,且芥花油组水平最高。综合考虑,这是由抗营养因子及高不饱和脂肪酸的缺乏共同导致的。
3.4 植物原料替代鱼粉鱼油对大菱鲆幼鱼抗氧化及非特异性免疫能力的影响
AKP及ACP被认为是机体免疫系统中巨噬细胞溶酶体的标志酶,且其本身也是非特异性免疫的重要水解酶,不仅可以水解入侵的病原体,而且可以促进吞噬细胞的吞噬及降解作用[40]。本试验中,各组血清中ACP活性无显著性差异,芥花油组及豆油组AKP活性显著提高。这与Li等[35]和Ray等[41]的报道一致,但Wang等[42]利用棉籽粕替代凡纳滨对虾Litopenaeus vannamei饲料中鱼粉的结果显示,当替代水平低于50%时,AKP及ACP活性与对照组并无显著性差异,但当替代水平进一步提高,这两种酶活性显著下降,由此推测,当替代水平较低时,养殖鱼能够应对饲料所引起的内环境变化,而当替代水平进一步提高,突破鱼机体自我调控能力时,表现出酶活性的降低。因此,试验对象应对植物原料所引起的内环境变化的自我调控能力是有限的,这一能力因鱼种、替代水平及抗营养因子含量而异。本试验中使用多种植物蛋白源,尽可能避免使用单一原料而导致抗营养因子含量过高,从存活率来看,试验对象对原料所引起的内环境的变化在其可自我调控范围内。
SOD、CAT和GSH-Px是生物体内广泛存在的抗氧化酶,可以有效清除活性氧自由基以保护机体组织免受损伤。LZM是鱼体非特异性免疫系统中重要的酶,能保护机体免受外源微生物的入侵。MDA是脂肪氧化的终产物之一,在体内蓄积会使得机体被活性氧族损伤。本试验中,各试验组在血清SOD、CAT、GSH-Px活性及MDA含量上无显著性差异,肝脏中SOD活性也无显著性差异,芥花油组肝脏MDA含量显著高于鱼粉鱼油组,而豆油组则在肝脏GSH-Px活性及MDA含量上与鱼粉鱼油组无显著性差异。各处理组肝脏中CAT活性显著低于鱼粉鱼油组,且无论血清或者肝脏中LZM水平在各处理组中均低于鱼粉鱼油组。以上结果与对真鲷[1]和星斑川鲽[38]饲料中鱼粉替代的研究结果类似。Wang等[3]在利用亚麻籽油替代大菱鲆日粮中鱼油的研究中也发现,植物油替代鱼油会对鱼体免疫能力造成负面影响,而Li等[32]对金鲳的研究表明,n-3HUFA有助于提高鱼体抗氧化能力。本试验中,在鱼粉及鱼油均被替代的情况下,芥花油组n-3PUFA的缺乏是导致该组抗氧化能力下降的主要原因,而豆油组添加裂壶藻及拟微绿球藻有效提高了该组n-3PUFA的含量,该组抗氧化能力优于芥花油组。此外,本研究结果也表明,植物原料替代鱼粉鱼油会对养殖鱼的非特异免疫能力造成负面影响,这可能也与某些氨基酸缺乏相关。饲料中适宜水平的精氨酸有利于提高养殖鱼的抗氧化和非特异免疫能力,Zhang等[43]对大菱鲆的研究发现,精氨酸对试验鱼生长并无促进作用,但可提高其非特异免疫及抗病能力。本试验处理组虽补充了晶体精氨酸,但由于饲料制作过程中的损失,以及晶体氨基酸和结合态氨基酸消化吸收不同步,造成了精氨酸在体内代谢过程中处于相对缺乏的状态,从而对处理组非特异性免疫能力造成了负面影响。
4 结论
1)不同植物性原料替代鱼粉鱼油对大菱鲆生长性能影响程度不一。豆油组生长性能与鱼粉鱼油组接近,优于芥花油组及鱼油组,混合植物蛋白配合豆油及藻粉有望成为替代鱼粉鱼油的理想饲料。
2)植物原料替代鱼粉鱼油会影响大菱鲆全鱼及肝脏氨基酸和脂肪酸组成。氨基酸方面,主要是某些必需氨基酸含量的降低;脂肪酸组成直接反映出饲料脂肪酸的特点,替代组大菱鲆全鱼及肝脏中DHA、EPA含量明显下降。
3)植物原料替代鱼粉鱼油会对大菱鲆的抗逆能力带来一定影响。以植物蛋白源搭配豆油及混合藻粉的豆油组在生长性能及抗逆能力方面优于芥花油组及鱼油组。同时替代鱼粉鱼油,必需氨基酸如蛋氨酸、赖氨酸,某些脂肪酸的缺乏,以及抗营养因子的存在,是影响大菱鲆抗逆能力的主要因素。
4)通过调节全植物蛋白饲料中脂肪酸的组成及饲料质地,可有效提高大菱鲆对饲料的利用效率。
[1] DOSSOU S,KOSHIO S,ISHIKAWA M,et al.Effect of partial replacement of fish meal by fermented rapeseed meal on growth,immune response and oxidative condition of red sea bream juvenile,Pagrus major[J].Aquaculture,2018, 490:228-235.
[2] KOKOU F,SARROPOULOU E,COTOU E,et al.Effects of fish meal replacement by a soybean protein on growth,histology,selected immune and oxidative status markers of gilthead sea bream,Sparus aurata[J].Journal of the World Aquaculture Society,2015,46(2):115-128.
[3] WANG Q C,HE G,MAI K S.Modulation of lipid metabolism,immune parameters,and hepatic transferrin expression in juvenile turbot (Scophthalmus maximus L.) by increasing dietary linseed oil levels[J].Aquaculture,2016,464,489-496.
[4] TORRECILLAS S,ROBAINA L,CABALLERO M J,et al.Combined replacement of fishmeal and fish oil in European sea bass (Dicentrarchus labrax):production performance,tissue composition and liver morphology[J].Aquaculture, 2017,474:101-112.
[5] SALZE G,MCLEAN E,BATTLE P R,et al.Use of soy protein concentrate and novel ingredients in the total elimination of fish meal and fish oil in diets for juvenile cobia,Rachycentron canadum[J].Aquaculture,2010,298(3/4):294-299.
[6] GATESOUPE F J,LEGER C,BOUDON M,et al.Lipid feeding of turbot (Scophthalmus maximus L.):influence on growth of supplementation with methyl esters of linolenic acid and fatty acids of ω9 series[J].Annales Dhydrobiologie,1977,8(2):247-254.
[7] QIAO H J,WANG J Y,ZHANG L M,et al.An improved direct transesterification method for fatty acid determination of Phaeodactylum tricornutum[J].Journal of Applied Phycology,2015,27(2):697-701.
[8] PANSERAT S,KOLDITZ C,RICHARD N,et al.Hepatic gene expression profiles in juvenile rainbow trout (Oncorhynchus mykiss) fed fishmeal or fish oil-free diets[J].The British Journal of Nutrition,2008,100(5):953-967.
[9] WATANABE T,AOKI H,SHIMAMOTO K,et al.A trial to culture yellowtail with non-fishmeal diets[J].Fisheries Science,1998,64(4):505-512.
[10] G MEZ-REQUENI P,MINGARRO M,CALDUCH-GINER J A,et al.Protein growth performance,amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata)[J].Aquaculture,2004,232(1/2/3/4):493-510.
MEZ-REQUENI P,MINGARRO M,CALDUCH-GINER J A,et al.Protein growth performance,amino acid utilisation and somatotropic axis responsiveness to fish meal replacement by plant protein sources in gilthead sea bream (Sparus aurata)[J].Aquaculture,2004,232(1/2/3/4):493-510.
[11] ZHANG C X,RAHIMNEJAD S,WANG Y R,et al.Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus):effects on growth,digestive enzymes activity,gut histology,and expression of gut inflammatory and transporter genes[J].Aquaculture,2018,483:173-182.
[12] ANDREW J E,HOLM J,HUNTINGFORD F A.The effect of pellet texture on the feeding behaviour of gilthead seabream (Sparus aurata L.)[J].Aquaculture,2004,232(1/2/3/4):471-479.
[13] AAS T S,SIXTEN H J,HILLESTAD M,et al.Measurement of gastrointestinal passage rate in Atlantic salmon (Salmo salar) fed dry or soaked feed[J].Aquaculture Reports,2017,8:49-57.
[14] GAO Z Y,WANG X,TAN C,et al.Effect of dietary methionine levels on growth performance,amino acid metabolism and intestinal homeostasis in turbot (Scophthalmus maximus L.)[J].Aquaculture,2019, 498:335-342.
[15] PERES H,OLIVA-TELES A.Lysine requirement and efficiency of lysine utilization in turbot (Scophthalmus maximus) juveniles[J].Aquaculture,2008,275(1/2/3/4):283-290.
[16] YANG M X,DENG K Y,PAN M Z,et al.Glucose and lipid metabolic adaptations during postprandial starvation of Japanese flounder Paralichthys olivaceus previously fed different levels of dietary carbohydrates[J].Aquaculture,2019,501:416-429.
[17] LI H Y,XU W J,JIN J Y,et al.Effects of starvation on glucose and lipid metabolism in gibel carp (Carassius auratus gibelio var. CAS III)[J].Aquaculture,2018,496:166-175.
[18] ROLLAND M,LARSEN B K,HOLM J,et al.Effect of plant proteins and crystalline amino acid supplementation on postprandial plasma amino acid profiles and metabolic response in rainbow trout (Oncorhynchus mykiss)[J].Aquaculture International,2015,23(4):1071-1087.
[19] TANTIKITTI C,MARCH B E.Dynamics of plasma free amino acids in rainbow trout (Oncorhynchus mykiss) under variety of dietary conditions[J].Fish Physiology and Biochemistry,1995,14(3):179-194.
[20] SKIBA-CASSY S,GEURDEN I,PANSERAT S,et al.Dietary methionine imbalance alters the transcriptional regulation of genes involved in glucose,lipid and amino acid metabolism in the liver of rainbow trout (Oncorhynchus mykiss)[J].Aquaculture,2016,454:56-65.
[21] PANSERAT S,HORTOPAN G A,PLAGNES-JUAN E,et al.Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver[J].Aquaculture, 2009,294(1/2):123-131.
[22] DE SILVA S S,FRANCIS D S,TACON A G J.Fish oils in aquaculture[M]//Turchini G M,Ng W K,Tocher D R.Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds.Boca Raton:CRC Press,2011:1-20.
[23] 宋泽,彭雍博,宋悦凡,等.裂殖壶菌营养成分及其多糖特征分析[J].大连海洋大学学报,2019,34(2):247-251.
[24] GARC A-ORTEGA A,KISSINGER K R,TRUSHENSKI J T.Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus[J].Aquaculture,2016,452:1-8.
A-ORTEGA A,KISSINGER K R,TRUSHENSKI J T.Evaluation of fish meal and fish oil replacement by soybean protein and algal meal from Schizochytrium limacinum in diets for giant grouper Epinephelus lanceolatus[J].Aquaculture,2016,452:1-8.
[25] YIN X W,MIN W W,LIN H J,et al.Population dynamics,protein content,and lipid composition of Brachionus plicatilis fed artificial macroalgal detritus and Nannochloropsis sp.diets[J].Aquaculture,2013,380-383:62-69.
[26] OSWALD A T O,ISHIKAWA M,KOSHIO S,et al.Nutritional evaluation of Nannochloropsis powder and lipid as alternative to fish oil for kuruma shrimp,Marsupenaeus japonicus[J].Aquaculture,2019,504:427-436.
[27] QIAO H,WANG H,SONG Z,et al.Effects of dietary fish oil replacement by microalgae raw materials on growth performance,body composition and fatty acid profile of juvenile olive flounder,Paralichthys olivaceus[J]. Aquaculture Nutrition,2014,20(6):646-653.
[28] PENG M,XU W,MAI K S,et al.Growth performance,lipid deposition and hepatic lipid metabolism related gene expression in juvenile turbot (Scophthalmus maximus L.) fed diets with various fish oil substitution levels by soybean oil[J].Aquaculture,2014,433:442-449.
[29] WATSON A M,BARROWS F T,PLACE A R.Taurine supplemented plant protein based diets with alternative lipid sources for juvenile gilthead sea bream,Sparus aurata[J].Journal of Fisheries and Aquaculture,2013,4(1):56-66.
[30] WATSON A M,BARROWS F T,PLACE A R.Taurine supplementation of plant derived protein and n-3 fatty acids are critical for optimal growth and development of cobia,Rachycentron canadum[J].Lipids,2013,48(9):899-913.
[31] LI B S,WANG J Y,HUANG Y,et al.Effects of replacing fish oil with wheat germ oil on growth,fat deposition,serum biochemical indices and lipid metabolic enzyme of juvenile hybrid grouper (Epinephelus fuscoguttatus♀×Epinephelus lanceolatus♂)[J].Aquaculture,2019,505:54-62.
[32] LI M M,ZHANG M,MA Y C,et al.Dietary supplementation with n-3 high unsaturated fatty acids decreases serum lipid levels and improves flesh quality in the marine teleost golden pompano Trachinotus ovatus[J].Aquaculture,2020,516:734632.
[33] GLENCROSS B,BLYTH D,IRVIN S,et al.An evaluation of the complete replacement of both fishmeal and fish oil in diets for juvenile Asian seabass,Lates calcarifer[J].Aquaculture,2016,451:298-309.
[34] BHAT I A,RATHOR P K,MIR I N,et al.Toxicological evaluation and effective dose selection of eurycomanone,a quassinoid of Eurycoma longifolia plant in fishes[J].Aquaculture,2017,481:94-102.
[35] LI P Y,WANG J Y,SONG Z D,et al.Evaluation of soy protein concentrate as a substitute for fishmeal in diets for juvenile starry flounder (Platichthys stellatus)[J].Aquaculture,2015,448:578-585.
[36] YUAN X Y,LIU M Y,CHENG H H,et al.Replacing fish meal with cottonseed meal protein hydrolysate affects amino acid metabolism via AMPK/SIRT1 and TOR signaling pathway of Megalobrama amblycephala[J].Aquaculture,2019,510:225-233.
[37] BU X Y,CHEN A J,LIAN X Q,et al.An evaluation of replacing fish meal with cottonseed meal in the diet of juvenile Ussuri catfish Pseudobagrus ussuriensis:growth,antioxidant capacity,nonspecific immunity and resistance to Aeromonas hydrophila[J].Aquaculture,2017,479:829-837.
[38] SONG Z D,LI H Y,WANG J Y,et al.Effects of fishmeal replacement with soy protein hydrolysates on growth performance,blood biochemistry,gastrointestinal digestion and muscle composition of juvenile starry flounder (Platichthys stellatus)[J].Aquaculture,2014,426/427:96-104.
[39] COUTO A,KORTNER T M,PENN M,et al.Effects of dietary phytosterols and soy saponins on growth,feed utilization efficiency and intestinal integrity of gilthead sea bream (Sparus aurata) juveniles[J].Aquaculture,2014, 432:295-303.
[40] WU P,SHI J R,WANG Y L,et al.Effect of Rhodospirillum rubrum in effluent on disease resistance,intestinal microbiota of silver carp[J].Aquaculture,2019,513:734400.
[41] RAY G W,LIANG D Z,YANG Q H,et al.Effects of replacing fishmeal with dietary soybean protein concentrate (SPC) on growth,serum biochemical indices,and antioxidative functions for juvenile shrimp Litopenaeus vannamei[J].Aquaculture,2020,516:734630.
[42] WANG J X,ZHANG H T,YANG Q H,et al.Effects of replacing soybean meal with cottonseed meal on growth,feed utilization and non-specific immune enzyme activities for juvenile white shrimp,Litopenaeus vannamei[J].Aquaculture Reports,2020,16:100255.
[43] ZHANG K K,MAI K S,XU W,et al.Effects of dietary arginine and glutamine on growth performance,nonspecific immunity,and disease resistance in relation to arginine catabolism in juvenile turbot (Scophthalmus maximus L.)[J].Aquaculture,2017,468:246-254.