自2000年以来,刺参Apostichopus japonicus养殖业发展迅速,已成为中国渔业的重要经济种类[1]。而养殖过程中,刺参皮肤溃疡、口围肿胀等多种疾病的发生,阻碍了该行业可持续发展[1]。由化学药物控制刺参病害会导致病原体产生抗药性[2]。此外,海产品和环境中存在药物残留可增加人类健康风险[3]。因此,期望益生菌作为一种环境友好的饲料添加剂,以增强养殖刺参的防御机制[4-12]。
益生菌是一种有益于其供给宿主健康的微生物,实际生产中应用海洋酵母,如梅奇酵母属Metschnikowia、有孢汉逊酵母属Hanseniaspora和红酵母属Rhodotorula的一些种类能促进幼参的生长[9-10,13-15]。此外,酵母添加剂还可提高幼参肠道消化酶活力[9,13-14,16]并刺激其免疫反应[4,6,9,15-16]。同样,酵母益生菌也显示出增强幼参对病原体感染的抵抗力[4,6,9-10,15]。然而,有关海洋酵母对刺参体壁营养组成和肠道菌群影响的报道较少。为此,本研究中探讨了饲用内源海洋红酵母H26[17]对幼参生长、消化酶活力、体壁营养和脂肪酸组成及肠道菌群的影响,以期为海洋红酵母在刺参养殖中的应用提供科学依据。
1 材料与方法
1.1 材料
试验用健康幼参购自大连某商业刺参养殖场。
1.2 方法
1.2.1 含菌饲料制备及试验设计 红酵母H26从健康刺参呼吸树分离获得。将红酵母H26菌株接种于酵母膏胨葡萄糖液体培养基(蛋白胨20 g、酵母粉10 g、葡萄糖20 g、陈海水1000 mL,自然pH)中,25 ℃下振荡培养16 h,将菌悬液离心,细胞用生理盐水重悬,用血球计数板计数,然后将菌悬液添加到幼参基础饲料中,配方参考文献[4],制备含H26菌为105、107cells/g的饲料,饲料每天制备一次以保证菌株活力。
试验中将幼参暂养2周,选择大小相近体质量为(0.54±0.06)g的幼参,随机分配到9个盛有过滤海水的塑料桶中,每桶200头,用不同浓度的含菌饲料和基础饲料分别投喂3桶幼参, 每日投喂一次, 投喂量为其体质量的5%,每2天换水1/2,吸底,用气石充气。试验期间水温为16~24 ℃,pH为7.8~8.2,盐度为30。
1.2.2 生长试验 分别在投喂前、第4周和第8周对幼参体质量进行测定。从每个桶中随机取30头幼参,用纱布吸去体表水分后称量其体质量。特定生长率(%/d)计算公式为
特定生长率=(lnWt-lnW0)/t×100%。
其中:W0和Wt分别为初始体质量(g)和第t天时的体质量(g);t为试验时间(d)。
1.2.3 样品采集 在养殖第4周和第8周时取样,幼参取样前饥饿20 h排空肠道内容物。从每个桶中随机取50头幼参,取其体壁用液氮迅速冷冻后保存于冰箱(-80 ℃)中用于营养组成和脂肪酸分析。再从每个桶中随机取10头幼参用于肠道酵母菌计数、免疫指标、消化酶活力和肠道菌群分析。取体腔液共500 μL(每头幼参50 μL)加入等体积抗凝剂[18],混合均匀,取400 μL进行呼吸爆发活力和吞噬活力测定,剩余体腔液于4 ℃下离心,取体腔液上清液(记为CF);沉淀用体腔细胞等渗液[18]重悬,将重悬液在冰浴中用超声波破碎仪破碎,4 ℃下离心,取体腔细胞裂解液上清液(记为CLS),用于溶菌酶活力和酚氧化酶活力分析。
将10头幼参肠道合并后加入4倍体积无菌生理盐水,用匀浆器匀浆后取一部分用于酵母菌计数,剩余部分用液氮迅速冷冻后保存于冰箱(-80 ℃)中。将保存的肠道匀浆液在4 ℃下解冻,取一部分匀浆液加入等量的生理盐水用于消化酶活力测定,剩余样品于-20 ℃下保存,用于变性梯度凝胶电泳(DGGE)分析。
1.2.4 蛋白含量测定 采用考马斯亮蓝法[19]测定体腔液、CLS和肠道匀浆液上清液中总蛋白含量。
1.2.5 消化酶活力测定 采用试剂盒(购于南京建成科技有限公司)测定胃蛋白酶活力、胰蛋白酶活力、淀粉酶活力和脂肪酶活力[20]。
1.2.6 体壁营养和脂肪酸组成测定 参考文献[20]中的方法测定刺参体壁粗蛋白质、糖分、粗脂肪和灰分含量。采用气相色谱法[21]测定养殖第8周时刺参体壁脂肪酸组成。
1.2.7 免疫指标分析 参考文献[22]的方法测定体腔细胞的吞噬活力和呼吸爆发,以及CF和CLS中的溶菌酶活力和酚氧化酶活力。
1.2.8 肠道酵母菌计数 将肠道匀浆液梯度稀释,取100 μL涂布于酵母膏蛋白胨葡萄糖琼脂平板上,25 ℃下培养7 d,进行计数。
1.2.9 肠道菌群分析 采用DGGE对肠道菌群进行分析。使用试剂盒[基因组DNA快速抽提试剂盒(土壤),生工生物工程(上海)股份有限公司]提取肠道总DNA。细菌群落分析使用细菌特异引物对1392R-GC/1055F[23]进行16S rRNA基因PCR扩增,扩增程序同文献[24]。真菌菌群落分析使用引物对NL-1/NL-4和NL1-GC/LS2进行巢式PCR扩增26S rRNA基因[25]。将得到的PCR产物参考文献[24]中的方法进行DGGE分析,细菌使用含45%~60%梯度变性剂的6%聚丙烯酰胺进行凝胶;真菌使用含30%~60%梯度变性剂的8%聚丙烯酰胺进行凝胶。从DGGE凝胶中切割条带再次进行PCR扩增(不带GC),PCR产物由生工生物工程(上海)股份有限公司进行测序,将序列与GenBank序列数据库中的其他16S rRNA和26S rRNA基因序列进行比较(http://www.ncbi. nlm.nih.gov/BLAST)。
1.3 数据处理
试验数据采用SPSS 16.0软件进行统计分析。酵母菌数量采用独立样本t检验进行统计分析。采用单因素方差分析和Tukey多重比较对试验数据进行分析,显著性水平设为0.05。试验数据采用平均值±标准差(mean±S.D.)表示。
2 结果与分析
2.1 饲料中添加H26对幼参生长的影响
从表1可见:与饲喂基础饲料比较,幼参养殖4周时,饲喂含H26菌株为107cells/g饲料的幼参特定生长率显著增加(P<0.05),而第8周时则无显著性差异(P>0.05);而饲喂含H26菌株105cells/g饲料的幼参在试验过程中特定生长率虽有增加但无显著性差异(表1)。
表1 H26对幼参特定生长率和肠道消化酶活力的影响
Tab.1 Effects of H26 on specific growth rate and intestinal digestive enzyme activities of juvenile sea cucumber
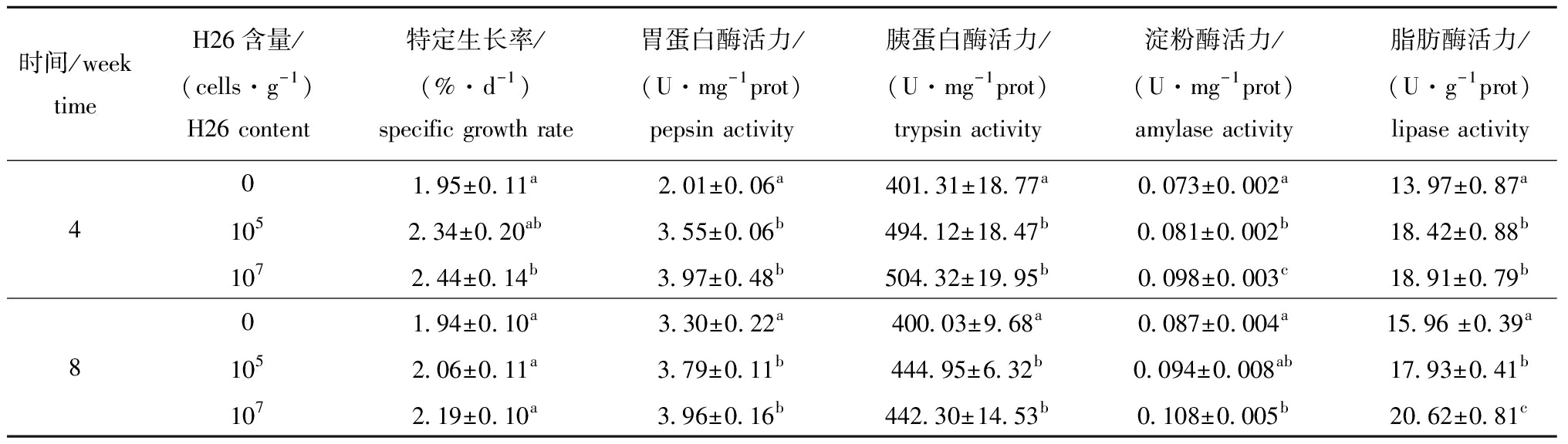
时间/weektimeH26含量/(cells·g-1)H26 content特定生长率/(%·d-1)specific growth rate胃蛋白酶活力/(U·mg-1prot)pepsin activity胰蛋白酶活力/(U·mg-1prot)trypsin activity淀粉酶活力/(U·mg-1prot)amylase activity脂肪酶活力/(U·g-1prot)lipase activity01.95±0.11a2.01±0.06a401.31±18.77a0.073±0.002a13.97±0.87a41052.34±0.20 ab3.55±0.06b494.12±18.47b0.081±0.002b18.42±0.88b1072.44±0.14 b3.97±0.48b504.32±19.95b0.098±0.003c18.91±0.79b01.94±0.10 a3.30±0.22a400.03±9.68a0.087±0.004a15.96 ±0.39a81052.06±0.11 a3.79±0.11b444.95±6.32b0.094±0.008ab17.93±0.41b1072.19±0.10 a3.96±0.16b442.30±14.53b0.108±0.005b20.62±0.81c
注:同列中标有不同字母者表示同一采样时间下不同组间有显著性差异(P<0.05),标有相同字母者表示组间无显著性差异(P>0.05),下同
Note:The means with different letters within the same column are significantly different in the groups at same sampling week at the 0.05 probability level, and the means with the same letters within the same column are not significant differences, et sequentia
2.2 饲料中添加H26对幼参肠道消化酶活力的影响
从表1还可见:幼参养殖第4周和第8周时,除饲喂含H26为 105cells/g饲料在第8周时幼参的淀粉酶活力外,所有饲喂含H26饲料幼参的胃蛋白酶、胰蛋白酶、淀粉酶和脂肪酶活力均显著高于对照组(P<0.05);饲喂含H26为 107cells/g饲料幼参在第4周时淀粉酶活力和第8周时脂肪酶活力显著高于饲喂含H26为105cells/g饲料的幼参(P<0.05),但饲喂含H26两种不同浓度的幼参胃蛋白酶和胰蛋白酶活力在第4周和第8周时均无显著性差异(P>0.05)。
2.3 饲料中添加H26对幼参体壁营养和脂肪酸组成的影响
从表2可见:幼参养殖第4周和第8周时,所有饲喂含H26饲料的幼参体壁粗蛋白质含量均显著高于对照组(P<0.05);饲喂含H26为 107cells/g饲料的幼参在第4周时及饲喂含H26为 105 、107cells/g饲料的幼参在第8周时体壁糖分含量均显著高于对照组(P<0.05);试验过程中,所有组幼参体壁粗脂肪含量均无显著性差异(P>0.05);饲喂含H26为 107cells/g饲料的幼参在第8周时体壁灰分含量显著低于对照组(P<0.05)。
从表3可见:养殖第8周时,花生四烯酸(C20:4n-6)是最丰富的脂肪酸(28.63 g/100 g),与饲喂基础饲料相比,饲喂含H26为 105、107cells/g饲料的幼参C20:4n-6含量显著提高(P<0.05),且饲喂含H26两种不同浓度的幼参C20:4n-6含量有显著性差异(P<0.05);饲喂含H26为 107cells/g饲料的幼参二高-γ-亚麻酸(C20:3n-6)和肾上腺酸(C22:4n-6)含量也显著高于饲喂基础饲料幼参(P<0.05);相反,与对照组相比,饲喂含H26为 105cells/g饲料的幼参二十四烯酸(C24:1)含量显著降低(P<0.05),饲喂含H26为 107cells/g饲料的幼参油酸(C18:1)、亚油酸(C18:2n-6)、二十碳二烯酸(C20:2n-6)+二十碳三烯酸(C20:3n-6)和二十碳五烯酸(C20:5n-3)含量也显著低于饲喂基础饲料和/或含H26为105cells/g饲料的幼参(P<0.05)。
表2 H26对幼参体壁营养组成的影响(干质量)
Tab.2 Effects of H26 on proximate composition (dry weight)in the body wall of juvenile sea cucumber w/%
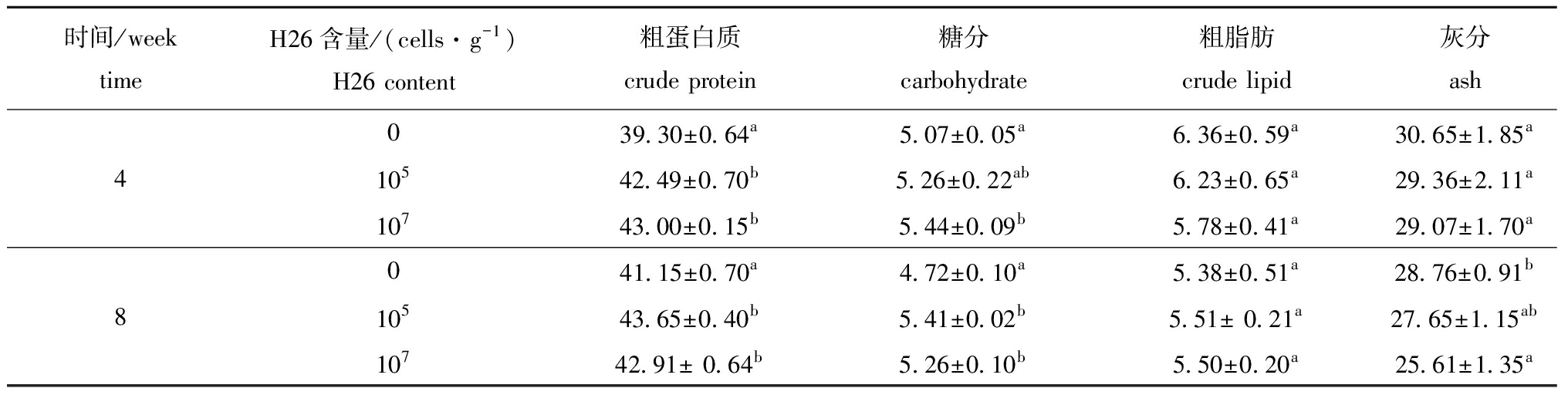
时间/weektimeH26含量/(cells·g-1)H26 content粗蛋白质crude protein糖分carbohydrate粗脂肪crude lipid灰分ash039.30±0.64a5.07±0.05a6.36±0.59a30.65±1.85a410542.49±0.70b5.26±0.22ab6.23±0.65a29.36±2.11a10743.00±0.15b5.44±0.09b5.78±0.41a29.07±1.70a041.15±0.70a4.72±0.10a5.38±0.51a28.76±0.91b810543.65±0.40b5.41±0.02b5.51± 0.21a27.65±1.15ab10742.91± 0.64b5.26±0.10b5.50±0.20a25.61±1.35a
表3 H26对幼参体壁脂肪酸组成的影响
Tab.3 Effects of H26 on fatty acids in the body wall of juvenile sea cucumberg/100 g
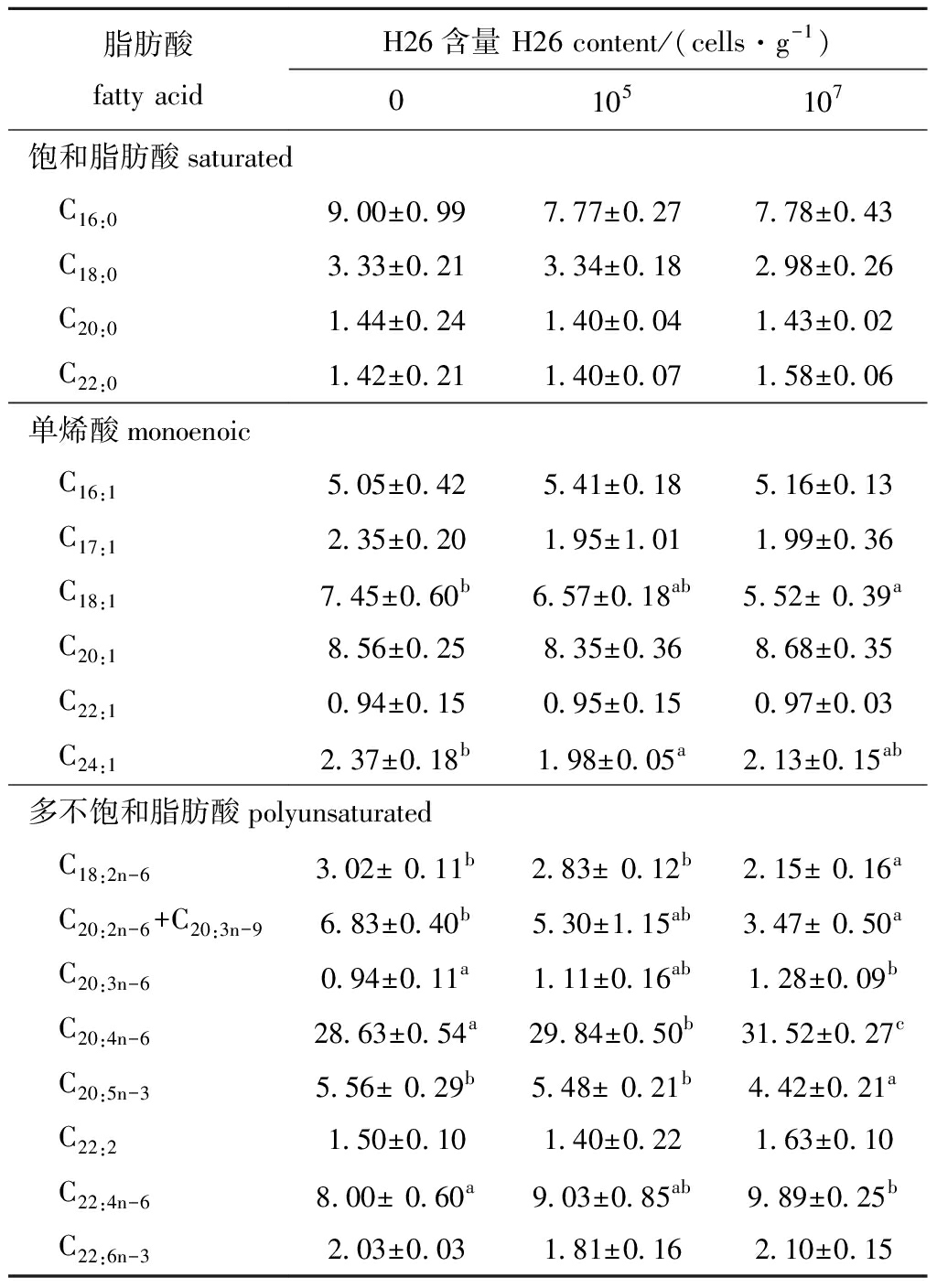
脂肪酸fatty acidH26含量 H26 content/(cells·g-1)0105107 饱和脂肪酸saturated C16:09.00±0.997.77±0.277.78±0.43 C18:03.33±0.213.34±0.182.98±0.26 C20:01.44±0.241.40±0.041.43±0.02 C22:01.42±0.211.40±0.071.58±0.06单烯酸monoenoic C16:15.05±0.425.41±0.185.16±0.13 C17:12.35±0.201.95±1.011.99±0.36 C18:17.45±0.60b6.57±0.18ab5.52± 0.39a C20:18.56±0.258.35±0.368.68±0.35 C22:10.94±0.150.95±0.150.97±0.03 C24:12.37±0.18b1.98±0.05a2.13±0.15ab多不饱和脂肪酸polyunsaturated C18:2n-63.02± 0.11b2.83± 0.12b2.15± 0.16a C20:2n-6+C20:3n-96.83±0.40b5.30±1.15ab3.47± 0.50a C20:3n-60.94±0.11a1.11±0.16ab1.28±0.09b C20:4n-628.63±0.54a29.84±0.50b31.52±0.27c C20:5n-35.56± 0.29b5.48± 0.21b4.42±0.21a C22:21.50±0.101.40±0.221.63±0.10 C22:4n-68.00± 0.60a9.03±0.85ab9.89±0.25b C22:6n-32.03±0.031.81±0.162.10±0.15
2.4 饲料中添加H26对幼参免疫指标的影响
从表4可见:幼参养殖第4周和第8周时,饲喂含H26为 107cells/g饲料的幼参体腔细胞吞噬活力显著高于对照组(P<0.05),而饲喂含H26为105cells/g饲料幼参则与对照组无显著性差异(P>0.05);所有饲喂含H26饲料的幼参在第4周和第8周时体腔细胞呼吸爆发较对照组显著增加(P<0.05)。
从表4还可见:幼参养殖第4周和第8周时,与饲喂基础饲料幼参相比,饲喂含H26为 107cells/g饲料的幼参具有较高的CF溶菌酶活力(P<0.05),而饲喂含H26为105cells/g饲料的幼参CF溶菌酶活力则无显著性差异(P>0.05);所有饲喂含H26饲料第4周和第8周后幼参CLS溶菌酶活力均显著高于饲喂基础饲料幼参(P<0.05);养殖第4周时,饲喂含H26为 105 、107cells/g饲料的幼参CF和CLS酚氧化酶活力均显著高于对照组(P<0.05);饲喂含H26为 107cells/g饲料4周后,幼参CF溶菌酶活力及CLS溶菌酶和酚氧化酶活力均显著高于饲喂含H26为 105 cells/g饲料的幼参(P<0.05),然而,饲喂8周后,各组间幼参CF和CLS酚氧化酶活力均无显著性差异(P>0.05)。
表4 H26对幼参体腔细胞吞噬活力和呼吸爆发及体腔液、体腔细胞裂解液上清液中溶菌酶和酚氧化酶活力的影响
Tab.4 Effects of H26 on phagocytic activity and respiratory burst in coelomocytes, and lysozyme and phenoloxidase activities in coelomic fluid(CF) and coelomocyte lysate supernatant (CLS) of juvenile sea cucumber
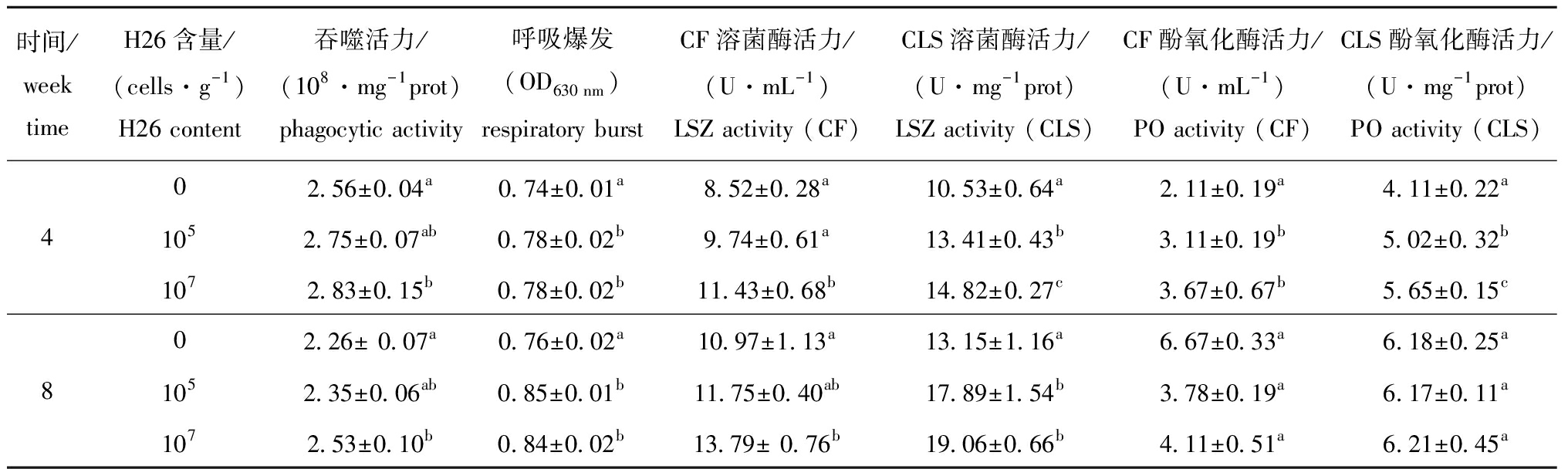
时间/weektimeH26含量/(cells·g-1)H26 content吞噬活力/(108·mg-1prot)phagocytic activity呼吸爆发(OD630 nm)respiratory burstCF溶菌酶活力/(U·mL-1) LSZ activity (CF)CLS溶菌酶活力/(U·mg-1prot)LSZ activity (CLS)CF酚氧化酶活力/(U·mL-1) PO activity (CF)CLS酚氧化酶活力/(U·mg-1prot)PO activity (CLS)02.56±0.04a0.74±0.01a8.52±0.28a10.53±0.64a2.11±0.19a4.11±0.22a41052.75±0.07ab0.78±0.02b9.74±0.61a13.41±0.43b3.11±0.19b5.02±0.32b1072.83±0.15b0.78±0.02b11.43±0.68b 14.82±0.27c3.67±0.67b5.65±0.15c02.26± 0.07a0.76±0.02a10.97±1.13a13.15±1.16a6.67±0.33a 6.18±0.25a81052.35±0.06ab0.85±0.01b11.75±0.40ab17.89±1.54b3.78±0.19a6.17±0.11a1072.53±0.10b0.84±0.02b13.79± 0.76b19.06±0.66b4.11±0.51a6.21±0.45a
2.5 H26在幼参肠道的存活
幼参养殖第4周和第8周时,从饲喂含H26为 105 、107cells/g饲料的幼参肠道中只能分离出H26,而对照组幼参肠道中未分离出酵母菌。此外,饲喂含H26为 107cells/g饲料第4周和第8周时,幼参肠道中H26细胞数量[分别为(1.35±0.09)× 104和(9.3±0.6)× 103cfu/g肠道]显著高于饲喂含H26为 105cells/g饲料的幼参[分别为(1.06±0.06) × 104 和(7.6±0.6)× 103cfu/g肠道]。
2.6 饲料中添加H26对幼参肠道菌群的影响
DGGE图谱显示,饲喂含H26饲料和基础饲料幼参肠道细菌群落组成相似(图1)。对从DGGE条带获得的16S rRNA基因序列进行BLAST分析,将序列分为3个纲(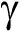 -、
-、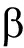 -和
-和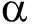 -变形菌纲)和未分类细菌(表5)。存在于所有组的条带1~2和4~7分别属于无色杆菌属、新鞘氨醇杆菌属、假交替单胞菌属、鞘氨醇属和弧菌属(图1,表5)。至于真菌群落,同一组3个平行样的DGGE图谱略有不同(图2),然而,纯培养H26条带只存在于饲喂含H26饲料的幼参肠道样品中。DGGE谱带的真菌26S rRNA基因序列鉴定为2个门(担子菌门和子囊菌门)和4个属(Meyerozyma、Pseudozyma、红酵母属、耶罗维亚酵母属)(图2,表6)。
-变形菌纲)和未分类细菌(表5)。存在于所有组的条带1~2和4~7分别属于无色杆菌属、新鞘氨醇杆菌属、假交替单胞菌属、鞘氨醇属和弧菌属(图1,表5)。至于真菌群落,同一组3个平行样的DGGE图谱略有不同(图2),然而,纯培养H26条带只存在于饲喂含H26饲料的幼参肠道样品中。DGGE谱带的真菌26S rRNA基因序列鉴定为2个门(担子菌门和子囊菌门)和4个属(Meyerozyma、Pseudozyma、红酵母属、耶罗维亚酵母属)(图2,表6)。
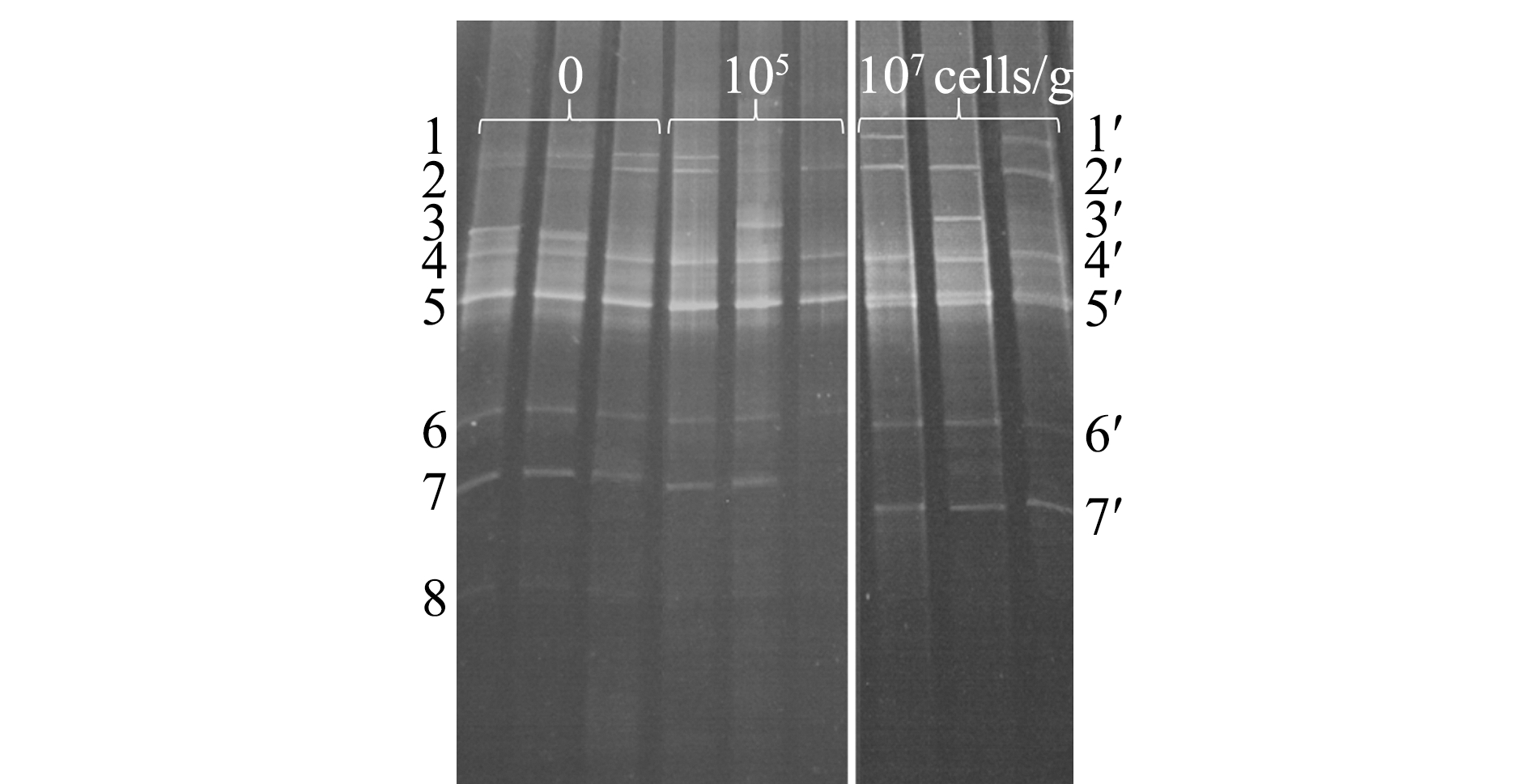
图1 饲喂基础饲料和含H26饲料幼参肠道细菌部分16S rRNA基因片段的DGGE图谱
Fig.1 DGGE profiles of the partial 16S rRNA gene fragments of the bacteria in the intestine of sea cucumber fed the control diet and H26-supplemented diets
表5 幼参肠道样品DGGE条带序列与GenBank数据库中最相似16S rRNA基因序列
Tab.5 Closest matches of 16S rRNA gene sequences in GenBank database to DGGE bands of intestinal samples in juvenile sea cucumber
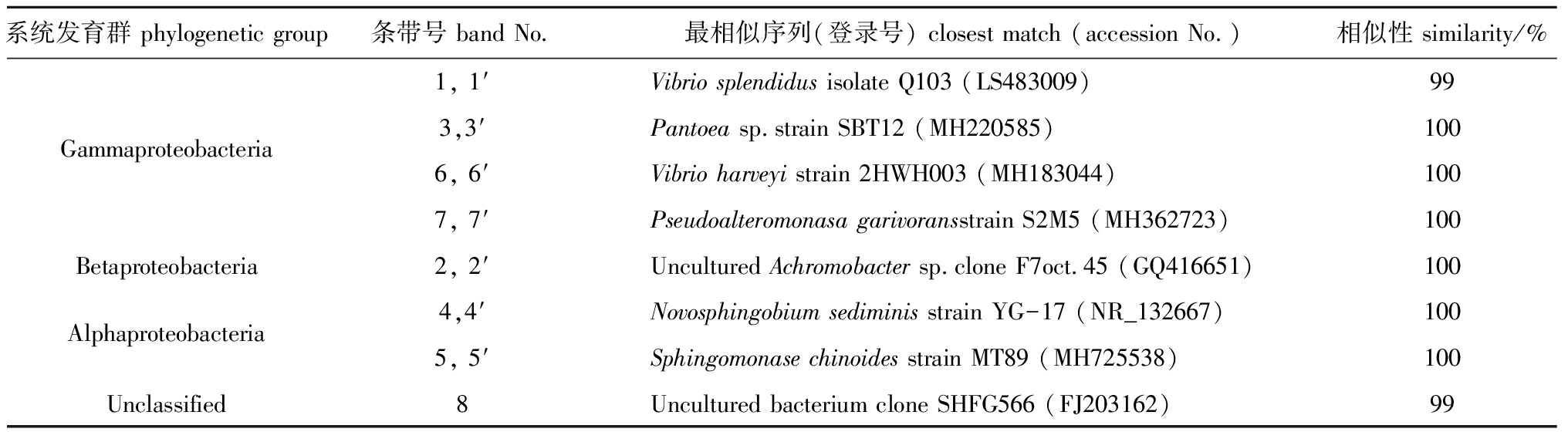
系统发育群 phylogenetic group条带号 band No.最相似序列(登录号) closest match (accession No.)相似性 similarity/%1, 1′Vibrio splendidus isolate Q103 (LS483009)99Gammaproteobacteria3,3′Pantoea sp.strain SBT12 (MH220585)1006, 6′Vibrio harveyi strain 2HWH003 (MH183044)1007, 7′Pseudoalteromonasa garivoransstrain S2M5 (MH362723)100Betaproteobacteria2, 2′Uncultured Achromobacter sp.clone F7oct.45 (GQ416651)100Alphaproteobacteria4,4′Novosphingobium sediminis strain YG-17 (NR_132667)1005, 5′Sphingomonase chinoides strain MT89 (MH725538)100Unclassified8Uncultured bacterium clone SHFG566 (FJ203162)99
表6 幼参肠道样品DGGE条带序列与GenBank数据库中最相似26S rRNA基因序列
Tab.6 Closest matches of 26S rRNA genes equences in GenBank database to DGGE bands of intestinal samples in juvenile sea cucumber

系统发育群 phylogenetic group条带号 band No.最相似序列(登录号) closest match (accession No.)相似性 similarity/%1, 1′Uncultured Pseudozyma clone S23SP03(M114641)99Basidiomycota4Rhodotorula mucilaginosa isolate 11(MH636067)1005, 5′Rhodotorula mucilaginosa isolate 8(MH636044)100Ascomycota2, 2′Meyerozyma guilliermondii isolate 35 (MH656392)1003Yarrowia lipolytica isolate MS29 (JN021559)100
3 讨论
3.1 H26对幼参生长、消化酶活力、体壁营养和脂肪酸组成的影响
海洋红酵母作为饲料添加剂对凡纳滨对虾Litopenaeus vannamei[26]、卵形鲳鯵Trachinotus ovatus[27]和刺参[9-10,15]均有良好的促生长作用。本研究中,饲料中添加H26为107 cells/g可显著提高幼参特定生长率,部分原因可能是酵母本身富含营养,如蛋白质、糖、核酸和维生素,可作为动物的营养增强剂[28]。与前人的研究结果[9,16]一致,本试验结果表明,H26能显著提高幼参的消化酶活力,可能与H26分泌胞外蛋白酶、脂肪酶和淀粉酶(未发表资料)从而提高幼参消化酶活力有关。此外,在饲料中添加H26可增加幼参体壁粗蛋白质和糖分含量,原因可能是,酵母胞外酶补充幼参酶活力,促进了饲料中复杂蛋白质和糖的消化,这样其易被宿主同化,最终导致幼参体壁中较高的蛋白质和糖积累。而且,饲喂含H26为 107 cells/g饲料的幼参体壁中C20:3n-6、C20:4n-6和C22:4n-6含量显著增加,原因可能是,该酵母可能提高了饲料利用率,有助于促进饲料中C20:3n-6、C20:4n-6和C22:4n-6的吸收和/或促进幼参从C18:2n-6合成C20:3n-6、C20:4n-6和C22:4n-6。不过,海洋红酵母和体壁C20:3n-6、C20:4n-6和C22:4n-6含量增加之间的关系需进一步研究。
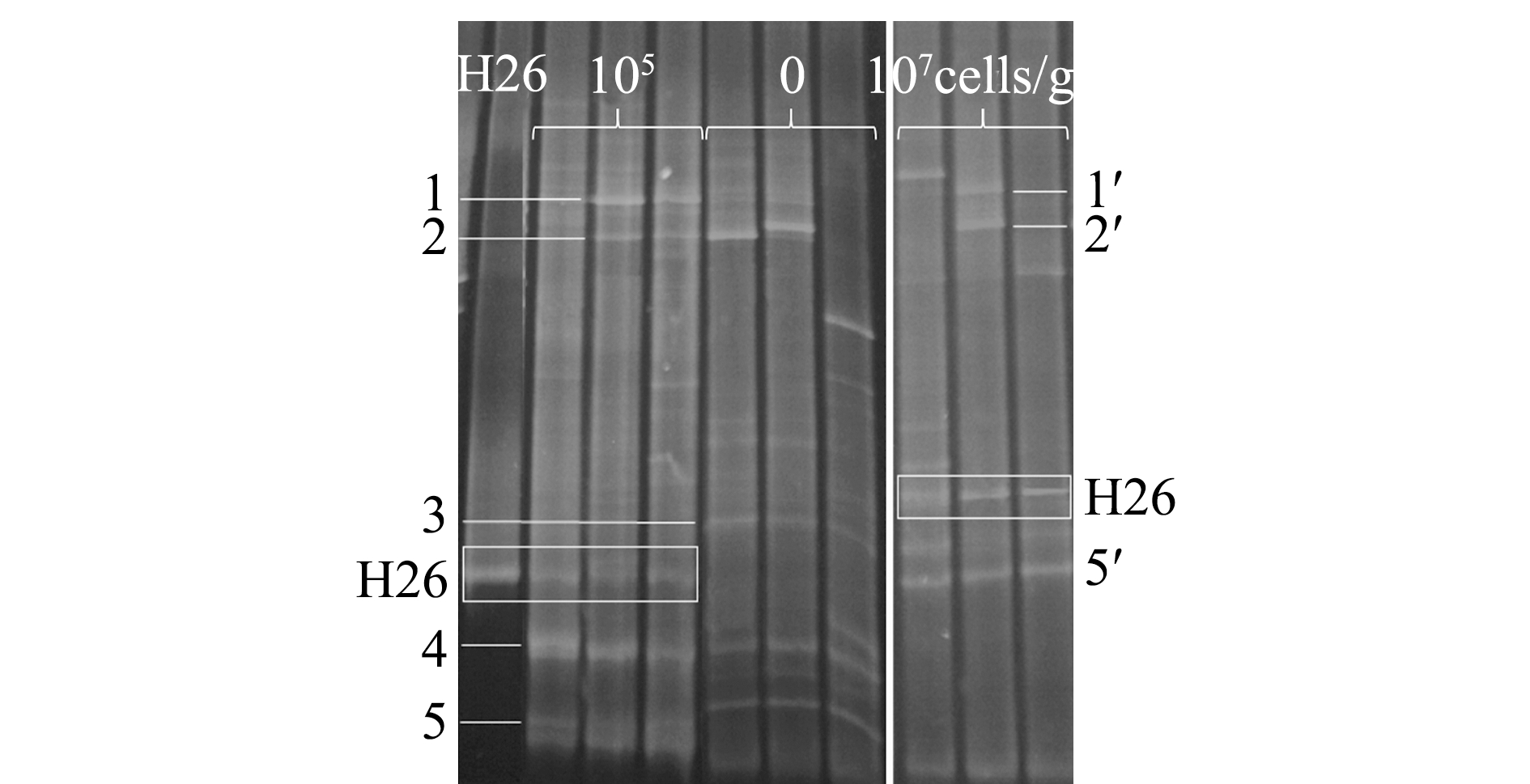
注:最左边泳道表示从纯培养纯化DNA扩增的H26条带;矩形框里面的带测序为红酵母H26
Note: Lane on the far left indicates H26, which is amplified usingpurified DNA from the pure culture;The bands enclosed by the rectangle are sequenced as Rhodotorula sp.H26
图2 饲喂基础饲料和含H26饲料幼参肠道真菌部分26S rRNA基因片段的DGGE图谱
Fig.2 DGGE profiles of the partial 26S rRNA gene fragments of the fungi in the intestine of sea cucumber fed the control diet and H26-supplemented diets
3.2 H26对幼参免疫指标的影响
有研究表明,海洋酵母影响水生动物的吞噬活力、呼吸爆发、溶菌酶和酚氧化酶活力等非特异性免疫指标[4-5,9,15,29]。棘皮动物体腔细胞起着吞噬、诱捕和包裹入侵微生物的作用[30-31]。在吞噬过程中,体腔细胞从呼吸爆发中产生杀微生物活性氧[32]。溶菌酶抑制革兰氏阳性和革兰氏阴性细菌的生长,酚氧化酶免疫系统在海参抗革兰氏阴性菌和双链RNA病毒感染中起重要作用[33]。研究表明,口服海洋红酵母可提高幼参体腔细胞的吞噬作用[9,16],增加CF和/或CLS的溶菌酶活力和/或酚氧化酶活力[9,15-16]。本试验中,饲料中添加H26能刺激幼参体腔细胞的吞噬活力和呼吸爆发,提高CF和CLS溶菌酶和酚氧化酶活力。根据口服β-葡聚糖和/或核苷酸可以增强幼参体腔细胞的吞噬活力和/或呼吸爆发[34-37],以及提高CLS溶菌酶[34]和酚氧化酶活力[36],推测本研究中酵母菌细胞组分(如葡聚糖或核苷酸)可能有助于免疫刺激作用。
3.3 H26对幼参肠道菌群的影响
H26最初是从天然水域采集的成参中分离获得的[17]。本研究中从试验幼参肠道中未检测到H26。饲喂4~8周后,H26只能从摄取含H26饲料幼参肠道中分离出。这表明通过饲喂引入的H26能够存活和耐受幼参肠道条件。研究证明,假交替单胞菌(图1条带7)在刺参[38]和欧洲鲍Haliotis tuberculata[39]中具有益生菌的潜能,而弧菌(图1条带1和6)作为条件致病菌与幼参急性口周肿胀病[40]、虾夷扇贝Patinopecten yessoensis及紫扇贝Argopecten purpuratus幼体大量死亡[41-42]有关。红酵母(图2条带4和5)和耶罗维亚酵母(图2条带3)可分别作为刺参[10,15-16]和巴拿马珠母贝珍珠Pinctada mazatlanica[43]的有效益生菌。总体来看,饲料中添加红酵母H26对幼参肠道菌群无显著影响。因此可认为,饲料中添加H26可能通过调节宿主肠道菌群平衡以实现对幼参生长、消化酶活力和先天性免疫指标的调节。
[1] Han Qingxi,Keesing J K,Liu Dongyan.A review of sea cucumber aquaculture,ranching,and stock enhancement in China[J].Reviews in Fisheries Science & Aquaculture,2016,24(4):326-341.
[2] 王宗杰.海参养殖环境耐药菌分析及三株海洋新菌的分类鉴定[D].济南:山东大学,2015.
[3] Defoirdt T,Sorgeloos P,Bossier P.Alternatives to antibiotics for the control of bacterial disease in aquaculture[J].Current Opinion in Microbiology,2011,14(3):251-258.
[4] Liu Zhiming,Ma Yuexin,Yang Zhiping,et al.Immune responses and disease resistance of the juvenile sea cucumber Apostichopus japonicus induced by Metschnikowia sp. C14[J].Aquaculture,2012,368-369:10-18.
[5] Zhao Yancui,Zhang Wenbing,Xu Wei,et al.Effects of potential probiotic Bacillus subtilis T13 on growth,immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus[J].Fish & Shellfish Immunology,2012,32(5):750-755.
[6] Ma Yuexin,Liu Zhiming,Yang Zhiping,et al.Effects of dietary live yeast Hanseniaspora opuntiae C21 on the immune and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus[J].Fish & Shellfish Immunology,2013,34(1):66-73.
[7] Yan Fajun,Tian Xiangli,Dong Shuanglin,et al.Growth performance,immune response,and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus fed a supplementary diet of the potential probiotic Paracoccus marcusii DB11[J].Aquaculture,2014,420-421:105-111.
[8] Li Jianguang,Xu Yongping,Jin Liji,et al.Effects of a probiotic mixture (Bacillus subtilis YB-1 and Bacillus cereus YB-2) on disease resistance and non-specific immunity of sea cucumber,Apostichopus japonicus (Selenka)[J].Aquaculture Research,2015,46(12):3008-3019.
[9] Wang Jihui,Zhao Liuqun,Liu Jinfeng,et al.Effect of potential probiotic Rhodotorula benthica D30 on the growth performance,digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus[J].Fish & Shellfish Immunology,2015,43(2):330-336.
[10] Yang Zhiping,Sun Jianming,Xu Zhe.Beneficial effects of Rhod- otorula sp. C11 on growth and disease resistance of juvenile Japanese spiky sea cucumber Apostichopus japonicus[J].Journal of Aquatic Animal Health,2015,27(2):71-76.
[11] Zhao Yancui,Yuan Lei,Wan Junli,et al.Effects of potential probiotic Bacillus cereus EN25 on growth,immunity and disease resistance of juvenile sea cucumber Apostichopus japonicus[J].Fish & Shellfish Immunology,2016,49:237-242.
[12] Liu Ningning,Zhang Shanshan,Zhang Weiwei,et al.Vibrio sp. 33 a potential bacterial antagonist of Vibrio splendidus pathogenic to sea cucumber (Apostichopus japonicus)[J].Aquaculture,2017,470:68-73.
[13] Ma Yuexin,Liu Zhiming,Yang Zhiping,et al.Effects of Hanseniaspora opuntiae C21 on the growth and digestive enzyme activity of juvenile sea cucumber Apostichopus japonicus[J].Chinese Journal of Oceanology and Limnology,2014,32(4):743-748.
[14] Yang Zhiping,Sun Jianming,Xu Zhe,et al.Beneficial effects of Metschnikowia sp. C14 on growth and intestinal digestive enzymes of juvenile sea cucumber Apostichopus japonicus[J].Animal Feed Science and Technology,2014,197:142-147.
[15] 张坤,刘金凤,董琦,等.饵料中添加胶红酵母对刺参性能的影响[J].大连工业大学学报,2017,36(1):6-9.
[16] 杨志平,徐哲,周倩,等.饵料中添加海洋红酵母(Rhodotorula sp.)C11对幼参消化酶及免疫反应的影响[J].渔业科学进展,2015,36(6):107-112.
[17] 李明,马悦欣,刘志明,等.刺参机体酵母菌组成及其拮抗活性的研究[J].大连海洋大学学报,2012,27(5):436-440.
[18] Xing Jun,Leung M F,Chia F S.Quantitative analysis of phagocytosis by amebocytes of a sea cucumber,Holothuria leucospilota[J].Invertebrate Biology,1998,117(1):67-74.
[19] Bradford M M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J].Analytical Biochemistry,1976,72(1-2):248-254.
[20] 包鹏云,李璐瑶,陈炜,等.饲料中添加混合益生菌对幼参生长、消化酶活力和体壁营养组成的影响[J].大连海洋大学学报,2018,33(1):52-56.
[21] Liao Mingling,Ren Tongjun,Chen Wei,et al.Effects of dietary lipid level on growth performance,body composition and digestive enzymes activity of juvenile sea cucumber,Apostichopus japonicus[J].Aquaculture Research,2017,48(1):92-101.
[22] 李璐瑶,孙丕海,包鹏云,等.饲料中添加混合益生菌对刺参幼参免疫反应和抗氧化性能的影响[J].大连海洋大学学报,2016,31(3):266-271.
[23] Ferris M J,Muyzer G,Ward D M.Denaturing gradient gel electrophoresis profiles of 16S rRNA-defined populations inhabiting a hot spring microbial mat community[J].Applied & Environmental Microbiology,1996,62:340-346.
[24] Ma Yuexin,Tao Wei,Liu Changfa,et al.Response of microbial biomass and bacterial community composition to fertilization in a salt marsh in China[J].Acta Oceanologica Sinica,2017,36(6):80-88.
[25] Prakitchaiwattana C J,Fleet G H,Heard G M.Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes[J].FEMS Yeast Research,2004,4(8):865-877.
[26] Yang Shiping,Wu Zaohe,Jian Jichang,et al.Effect of marine red yeast Rhodosporidium paludigenum on growth and antioxidant competence of Litopenaeus vannamei[J].Aquaculture,2010,309(1-4):62-65.
[27] Zhou Chuanpeng,Lin Heizhao,Xia Dongmei,et al.Effect of dietary marine red yeast Rhodotorula mucilaginosa on the growth performance,and also non-specific immune responses of juvenile golden pompano Trachinotus ovatus when challenged with Vibrio harveyi[J].Israeli Journal of Aquaculture-Bamidgeh,2016,68:1262.
[28] Brown M R,Barrett S M,Volkman J K,et al.Biochemical composition of new yeasts and bacteria evaluated as food for bivalve aquaculture[J].Aquaculture,1996,143(3-4):341-360.
[29] Sajeevan T P,Philip R,Singh I S B.Immunostimulatory effect of a marine yeast Candida sake S165 in Fenneropenaeus indicus[J].Aquaculture,2006,257(1-4):150-155.
[30] Glinski Z,Jarosz J.Immune phenomena in echinoderms[J].Archivum Immunologiae et Therapiae Experimentalis,2000,48(3):189-193.
[31] Chia F S,Xing J.Echinoderm coelomocytes[J].Zoological Studies,1996,35:231-254.
[32] Coteur G,Warnau M,Jangoux M,et al.Reactive oxygen species (ROS) production by amoebocytes of Asterias rubens (Echinodermata)[J].Fish & Shellfish Immunology,2002,12(3):187-200.
[33] Xue Zhuang,Li Hui,Wang Xiuli,et al.A review of the immune molecules in the sea cucumber[J].Fish & Shellfish Immunology,2015,44(1):1-11.
[34] Chang Jie,Zhang Wenbing,Mai Kangsen,et al.Effects of dietary β-glucan and glycyrrhizin on non-specific immunity and disease resistance of the sea cucumber (Apostichopus japonicus Selenka) challenged with Vibrio splendidus[J].Journal of Ocean University of China,2010,9(4):389-394.
[35] Gu Min,Ma Hongming,Mai Kangsen,et al.Effects of dietary β-glucan,mannan oligosaccharide and their combinations on growth performance,immunity and resistance against Vibrio splendidus of sea cucumber,Apostichopus japonicus[J].Fish & Shellfish Immunology,2011,31(2):303-309.
[36] Zhao Yancui,Ma Hongming,Zhang Wenbing,et al.Effects of dietary β-glucan on the growth,immune responses and resistance of sea cucumber,Apostichopus japonicus against Vibrio splendidus infection[J].Aquaculture,2011,315(3-4):269-274.
[37] Wei Zehong,Yi Lina,Xu Wei,et al.Effects of dietary nucleotides on growth,non-specific immune response and disease resistance of sea cucumber Apostichopus japonicus[J].Fish & Shellfish Immunology,2015,47(1):1-6.
[38] Ma Yuexin,Sun Feixue,Zhang Congyao,et al.Effects of Pseudoalteromonas sp. BC228 on digestive enzyme activity and immune response of juvenile sea cucumber (Apostichopus japonicus)[J].Journal of Ocean University of China,2014,13(6):1061-1066.
[39] Offret C,Rochard V,Laguerre H,et al.Protective efficacy of a Pseudoalteromonas strain in European abalone,Haliotis tuberculata,infected with Vibrio harveyi ORM4[J].Probiotics and Antimicrobial Proteins,2018,doi:10.1007/s12602-018-9389-8.
[40] 马悦欣,徐高蓉,张恩鹏,等.仿刺参幼参急性口围肿胀症的细菌性病原[J].水产学报,2006,30(3):377-382.
[41] Liu Jichen,Sun Xueying,Li Ming,et al.Vibrio infections associated with Yesso scallop (Patinopecten yessoensis) larval culture[J].Journal of Shellfish Research,2015,34(2):213-216.
[42] Rojas R,Miranda C D,Opazo R,et al.Characterization and pathogenicity of Vibrio splendidus strains associated with massive mortalities of commercial hatchery-reared larvae of scallop Argopecten purpuratus (Lamarck,1819)[J].Journal of Invertebrate Pathology,2015,124:61-69.
[43] Aguilar-Macías O L,Ojeda-Ramírez J J,Campa-Córdova  I,et al.Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster,Pinctada mazatlanica,during late hatchery and early field culturing[J].Journal of the World Aquaculture Society,2010,41(3):447-454.
I,et al.Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster,Pinctada mazatlanica,during late hatchery and early field culturing[J].Journal of the World Aquaculture Society,2010,41(3):447-454.