大豆分离蛋白替代鱼粉对哲罗鱼消化生理的影响
王常安,刘红柏,徐奇友,李晋南,王连生,赵志刚,尹家胜
(中国水产科学研究院黑龙江水产研究所, 黑龙江 哈尔滨 150070)
摘要:为研究大豆分离蛋白替代鱼粉后对哲罗鱼Huchotaimen消化系统组织结构和消化酶活性的影响,在水温为9.8~16.2 ℃时,将初始体质量为(6.90±0.04)g的哲罗鱼饲养于室内220 L玻璃钢水族箱中,分别投喂大豆分离蛋白替代0、25.0%、37.5%、50.0%、62.5%、75.0%、87.5%和100.0%鱼粉的饲料,每个处理设3个重复,每个重复放100尾鱼,以含有40.0%鱼粉的饲料为对照,试验时间为56 d。结果表明:随着鱼粉替代比例的增加,哲罗鱼胃、前肠、幽门盲囊、中肠和胰腺的蛋白酶、脂肪酶和淀粉酶活性均呈显著下降趋势(P<0.05);肠道绒毛和纹状缘高度亦呈显著下降趋势(P<0.05);高比例替代鱼粉水平(>75%)时,哲罗鱼肠道组织结构完整性被破坏,纹状缘融合、部分脱落,肝脏空泡化现象严重,形态轮廓逐渐模糊,肝细胞核偏移,且溶解或缺失。研究表明,大豆分离蛋白高比例替代鱼粉水平(>75%)显著降低消化系统消化酶活性,损伤肝脏和肠道组织。
关键词:哲罗鱼;大豆分离蛋白;消化酶;组织结构
目前,世界上2/3的鱼粉用来生产水产饲料[1],随着水产养殖业的发展,鱼粉的需求量逐渐增大,渔业资源的有限性推高了鱼粉的价格。为满足水产养殖业可持续发展的需要,常用植物蛋白替代水产饲料中一定比例的鱼粉[2-5]。大豆蛋白氨基酸较为平衡,分布广泛,成本较低,是优质的蛋白源[6]。然而,大豆蛋白中含有一些抗营养因子,如胰蛋白酶抑制因子、大豆球蛋白、皂甙等[7],致使消化率、适口性和利用率降低[8]。饲料中大豆蛋白含量较高时,鱼类的肠道、肝脏会发生炎症反应,甚至损伤组织结构[9-10],如大西洋鲑Salmosalar发生肠道绒毛变短,固有层和黏膜层肿胀,肠道上皮细胞空泡化等症状[11-12],但牙鲆Paralichthysolivaceus[13]等却未表现出明显的症状,因此,此种情况也具有种属差异性。
哲罗鱼Huchotaimen生长速度较快,经济价值较高,是中国珍稀名贵的土著鲑科鱼类。目前,有关其蛋白质[14]、脂肪[14]、肌醇[15]、磷[16]等营养素的需要量已确定,但关于其对原料利用的资料有限。哲罗鱼为肉食性鱼类,对蛋白质的需求量高,喜好动物蛋白。前期研究表明,大豆分离蛋白替代鱼粉后,其生长减缓,成活率和饲料利用率降低[17]。本试验在前期试验的基础上,研究了大豆分离蛋白替代鱼粉后对哲罗鱼肠道和肝脏的组织结构和消化酶活性的影响,旨在从消化生理方面解释其生长受抑制的原因,为配制其饲料提供参考。
1材料与方法
1.1材料
哲罗鱼初始体质量为(6.90±0.04)g。大豆分离蛋白购自哈尔滨高科技(集团)股份有限公司。选取体质健康、规格一致的哲罗鱼放入室内流水系统,用基础饲料驯养14 d后开展养殖试验。
1.2方法
1.2.1 试验饲料的配制 试验饲料以鱼粉、大豆分离蛋白、小麦面筋、玉米蛋白粉、血粉为蛋白源,以鱼油、豆油和磷脂为脂肪源。对照组G1饲料中含有40%鱼粉,处理组G2~G8饲料中以大豆分离蛋白替代25.0%、37.5%、50.0%、62.5%、75.0%、87.5%和100.0%的鱼粉,8种等氮等能试验饲料的配方及营养水平见表1。原料均过80目筛后用鼓型混合机混合,膨化制粒(直径为1.5 mm),于冰箱(-20 ℃)中保存待用。
表1试验饲料配方及营养水平(风干基础)
Tab.1Ingredientandapproximatecompositionofthebasicaldietusedintheexperiment(air-drybasis)w/%
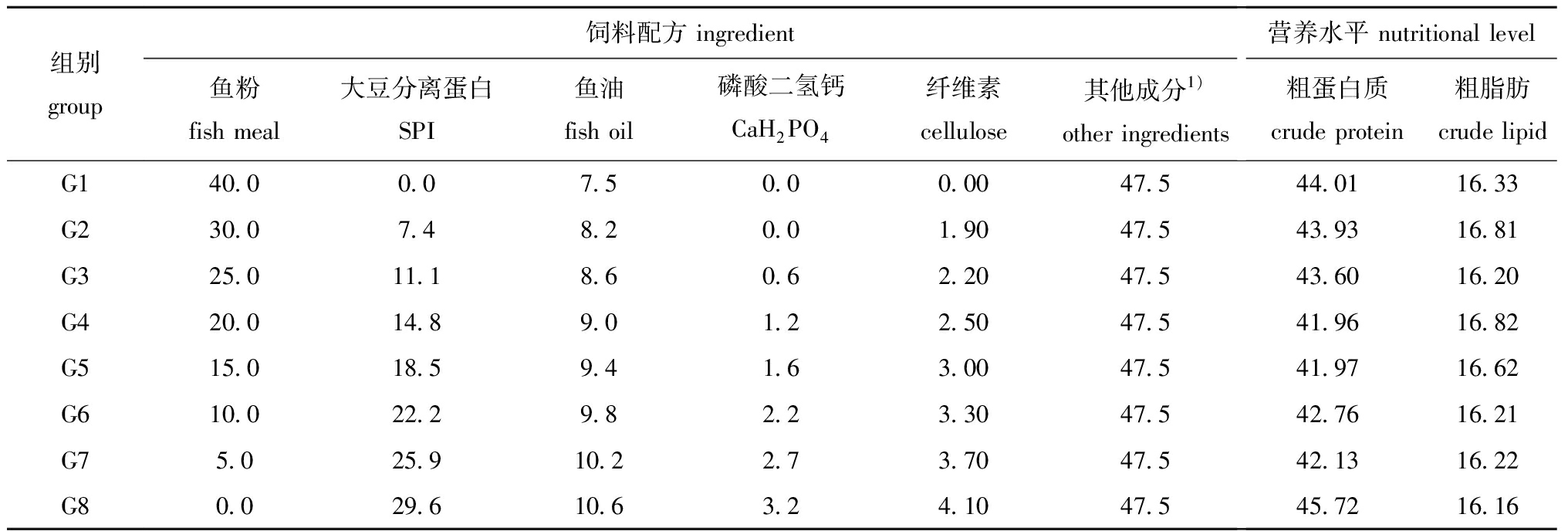
组别group饲料配方 ingredient 鱼粉fish meal大豆分离蛋白SPI鱼油fish oil磷酸二氢钙CaH2PO4纤维素cellulose其他成分1)other ingredients营养水平 nutritional level粗蛋白质crude protein粗脂肪crude lipidG140.00.07.50.00.0047.544.0116.33G230.07.48.20.01.9047.543.9316.81G325.011.18.60.62.2047.543.6016.20G420.014.89.01.22.5047.541.9616.82G515.018.59.41.63.0047.541.9716.62G610.022.29.82.23.3047.542.7616.21G75.025.910.22.73.7047.542.1316.22G80.029.610.63.24.1047.545.7216.16
注: 1)其他成分包括次粉22.3%、 血粉5.0%、 小麦面筋5.0%、 玉米蛋白10.0%、 豆油7.0%、 磷脂2.0%、 预混剂1.20%;2)预混剂包括甜菜碱0.1%、 胆碱0.2%、 防霉剂 0.05%、 抗氧化剂 0.025%、 VC 0.1%、 复合维生素0.1%、 复合微量元素0.5%; 3)维生素和微量元素包括VA 15 000 IU/kg、 VD33000 IU/kg、 VE 60 mg/kg、 VK35 mg/kg、 VB115 mg/kg、 VB230 mg/kg、 VB615 mg/kg、 VB120.5 mg/kg、 烟酸 175 mg/kg、 叶酸5 mg/kg、 泛酸钙 50 mg/kg、 生物素 2.5 mg/kg、 肌醇1000 mg/kg、 碘 0.6 mg/kg、 锰 15 mg/kg、 铜 3 mg/kg、 铁25 mg/kg
Note: 1)In each diet,the followings are supplied: wheat flour middling 22.3%, blood meal 5.0%,wheat gluten 5.0%, corn gluten meal 10.0%, soy oil 7.0%, soy lecithin 2.0%, premix 1.20%;2)Premix includes betaine 0.1%, choline 0.2%, antimildew 0.05%, antioxidant 0.025%, VC 0.1%, vitamin premix 0.1%; mineral premix 0.5%; 3)Vitamin premix and mineral premix include VA 15 000 IU/kg, VD33000 IU/kg, VE 60 mg/kg, VK35 mg/kg, VB115 mg/kg, VB230 mg/kg, VB615 mg/kg, VB120.5 mg/kg, nicotinic acid 175 mg/kg, folic acid 5 mg/kg, calcium pantothenate 50 mg/kg, biotin 2.5 mg/kg, inositol 1000 mg/kg, I 0.6 mg/kg, Mn 15 mg/kg, Cu 3 mg/kg, and Fe 25 mg/kg
1.2.2 试验设计及饲养管理 试验设8个处理组(G1~G8),每个处理组设3个重复,每个重复放100尾鱼。试验在室内玻璃钢水族箱(220 L)中进行。试验水为涌泉水,水温为9.8~16.2 ℃,溶氧>8.0 mg/L。日投喂2次,前4周投饲率为鱼体质量的3.4%,后4周投饲率为鱼体质量的2.0%,养殖周期为56 d。
1.2.3 样品采集 养殖试验结束后,将鱼饥饿24 h,用苯氧乙醇(0.05%)麻醉,采集样本。从每个重复随机取4尾鱼,于冰盘上取胃、肠道(剔除内含物和脂肪)和胰腺组织,用冰浴生理盐水(0.86%)洗净,滤纸吸干,称重。按质量体积比为1∶9(g∶mL)加入预冷的生理盐水,用FJ-200CL高速组织匀浆机匀浆3 min(15 000 r/min),在4 ℃条件下以4 000 r/min离心10 min,取上清液放入1.5 mL离心管中,于超低温冰箱(-80 ℃)中保存,用于测定前肠、中肠、后肠和胰腺的蛋白酶、脂肪酶和淀粉酶活性。
1.2.4 消化酶活性测定及组织切片观察 消化酶活性测定:分别采用福林-酚法、聚乙烯醇橄榄油乳化液水解法和淀粉-碘比色法测定蛋白酶、脂肪酶和淀粉酶活性[18]。
组织切片:从每个重复随机取4尾鱼的肝脏、前肠、中肠和幽门盲囊,在Bouin’s液中固定48 h,常规石蜡包埋,用KD1508型切片机横向连续切片,切片厚度为6 μm,用苏木精-伊红染色法染色,中性树胶封片,在Olympus光学显微镜下测定中肠的绒毛高度和纹状缘的高度。每张切片测定10个肠绒毛和纹状缘高度。
1.3数据处理
试验数据均以平均值±标准差(mean±S.D.)表示,采用SPSS for Windows 23.0软件进行单因素方差分析和Duncan’s多重比较,显著性水平设为0.05。
2结果与分析
2.1大豆分离蛋白替代鱼粉对哲罗鱼蛋白酶活性的影响
从表2可见:随着饲料中大豆分离蛋白质水平的增加,哲罗鱼胃、前肠+幽门盲囊、中肠和胰腺的蛋白酶活性明显下降;各鱼粉替代组鱼的胃、前肠+幽门盲囊蛋白酶活性均显著低于对照组(P<0.05),当鱼粉替代水平大于87.5%及以上时,中肠和胰腺蛋白酶活性均显著低于对照组(P<0.05)。
表2大豆分离蛋白替代鱼粉对哲罗鱼蛋白酶活性的影响
Tab.2EffectsoffishmealreplacementbysoybeanproteinisolateonproteaseactivitiesoftaimenHuchotaimen
U/g prot
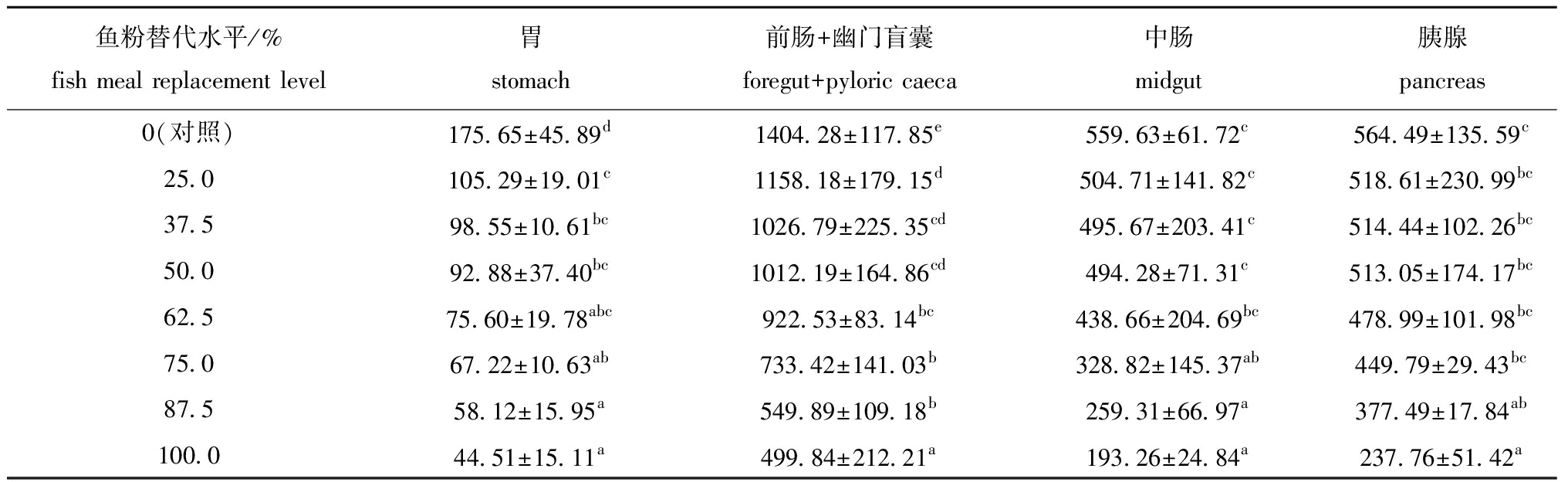
鱼粉替代水平/%fish meal replacement level胃stomach前肠+幽门盲囊foregut+pyloric caeca中肠midgut胰腺pancreas0(对照)175.65±45.89d1404.28±117.85e559.63±61.72c564.49±135.59c25.0105.29±19.01c1158.18±179.15d504.71±141.82c518.61±230.99bc37.598.55±10.61bc1026.79±225.35cd495.67±203.41c514.44±102.26bc50.092.88±37.40bc1012.19±164.86cd494.28±71.31c513.05±174.17bc62.575.60±19.78abc922.53±83.14bc438.66±204.69bc478.99±101.98bc75.067.22±10.63ab733.42±141.03b328.82±145.37ab449.79±29.43bc87.558.12±15.95a549.89±109.18b259.31±66.97a377.49±17.84ab100.044.51±15.11a499.84±212.21a193.26±24.84a237.76±51.42a
注:同列中标有不同小写字母者表示组间有显著性差异(P<0.05),标有相同小写字母者表示组间无显著性差异(P>0.05),下同
Note: The means with different letters within the same column are significantly different in the groups at the 0.05 probability level, and the means with the same letters within the same column are not significant differences, et sequentia
2.2大豆分离蛋白替代鱼粉对哲罗鱼脂肪酶活性的影响
从表3可见:随着饲料中大豆分离蛋白水平的增加,哲罗鱼胃、前肠+幽门盲囊、中肠和胰腺的脂肪酶活性明显下降;各鱼粉替代组鱼的前肠+幽门盲囊中脂肪酶活性显著低于对照组(P<0.05),当鱼粉替代水平为75.0%及以上时,胃、中肠、胰腺脂肪酶活性均显著低于对照组(P<0.05)。
表3大豆分离蛋白替代鱼粉对哲罗鱼脂肪酶活性的影响
Tab.3EffectsoffishmealreplacementbysoybeanproteinisolateonlipaseactivitiesoftaimenHuchotaimen
U/g prot
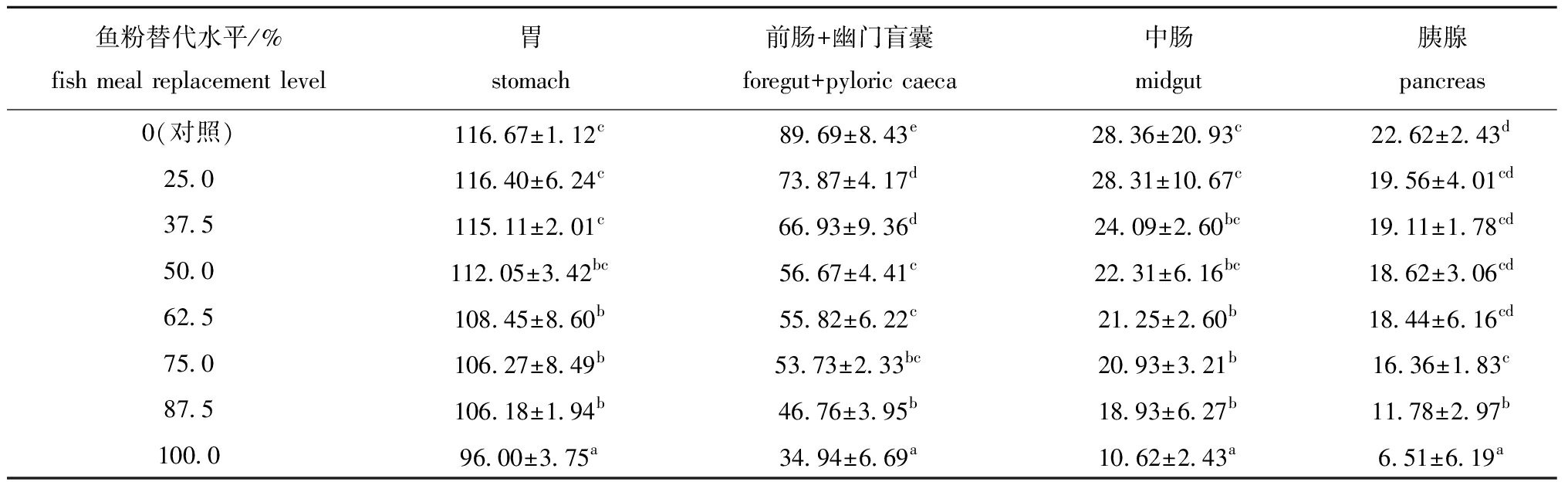
鱼粉替代水平/%fish meal replacement level胃stomach前肠+幽门盲囊foregut+pyloric caeca中肠midgut胰腺pancreas0(对照)116.67±1.12c89.69±8.43e28.36±20.93c22.62±2.43d25.0116.40±6.24c73.87±4.17d28.31±10.67c19.56±4.01cd37.5115.11±2.01c66.93±9.36d24.09±2.60bc19.11±1.78cd50.0112.05±3.42bc56.67±4.41c22.31±6.16bc18.62±3.06cd62.5108.45±8.60b55.82±6.22c21.25±2.60b18.44±6.16cd75.0106.27±8.49b53.73±2.33bc20.93±3.21b16.36±1.83c87.5106.18±1.94b46.76±3.95b18.93±6.27b11.78±2.97b100.096.00±3.75a34.94±6.69a10.62±2.43a6.51±6.19a
2.3大豆分离蛋白替代鱼粉对哲罗鱼淀粉酶活性的影响
从表4可见:随着饲料中大豆分离蛋白水平的增加,哲罗鱼胃、前肠+幽门盲囊、中肠和胰腺的淀粉酶活性明显下降;各鱼粉替代组鱼的胃淀粉酶活性显著低于对照组(P<0.05),当鱼粉替代水平为87.5%及以上时前肠+幽门盲囊、胰腺中淀粉酶活性显均著低于对照组(P<0.05)。
2.4大豆分离蛋白替代鱼粉对哲罗鱼消化组织形态的影响
从表5可见:随着饲料中大豆分离蛋白质水平的增加,哲罗鱼中肠绒毛和纹状缘高度明显下降;当鱼粉替代水平为37.5%及以上时,肠绒毛和纹状缘高度均显著低于对照组(P<0.05)。
对照组哲罗鱼肝细胞排列较为整齐,细胞界限明显,细胞核清晰可见(图1-A);随着大豆分离蛋白质水平的增加,肝脏组织结构呈现不同程度的破坏,出现病理现象(图1-B);替代水平为100.0%时,肝脏空泡化现象严重,形态轮廓逐渐模糊,肝细胞核发生偏移,部分逐渐溶解或缺失(图1-C)。观察发现,对照组哲罗鱼前肠、中肠和幽门盲囊的上皮组织结构完整,绒毛发达,上皮细胞排列整齐,杯状细胞较多(图1-D、G、J);随着鱼粉替代水平的增加,组织结构呈现不同程度的破坏,当鱼粉替代水平为75%时,开始出现病理现象,组织结构完整性被破坏,部分纹状缘融合、脱落(图1-E、H、K);替代水平为87.5%和完全替代时表现的病理特征类似(选取完全替代水平的病理切片图说明),组织结构被破坏,纹状缘脱落严重(图1-F、I、L)。
表4大豆分离蛋白替代鱼粉对哲罗鱼淀粉酶活性的影响
Tab.4EffectsoffishmealreplacementbysoybeanproteinisolateonamylaseactivitiesoftaimenHuchotaimen
U/g prot
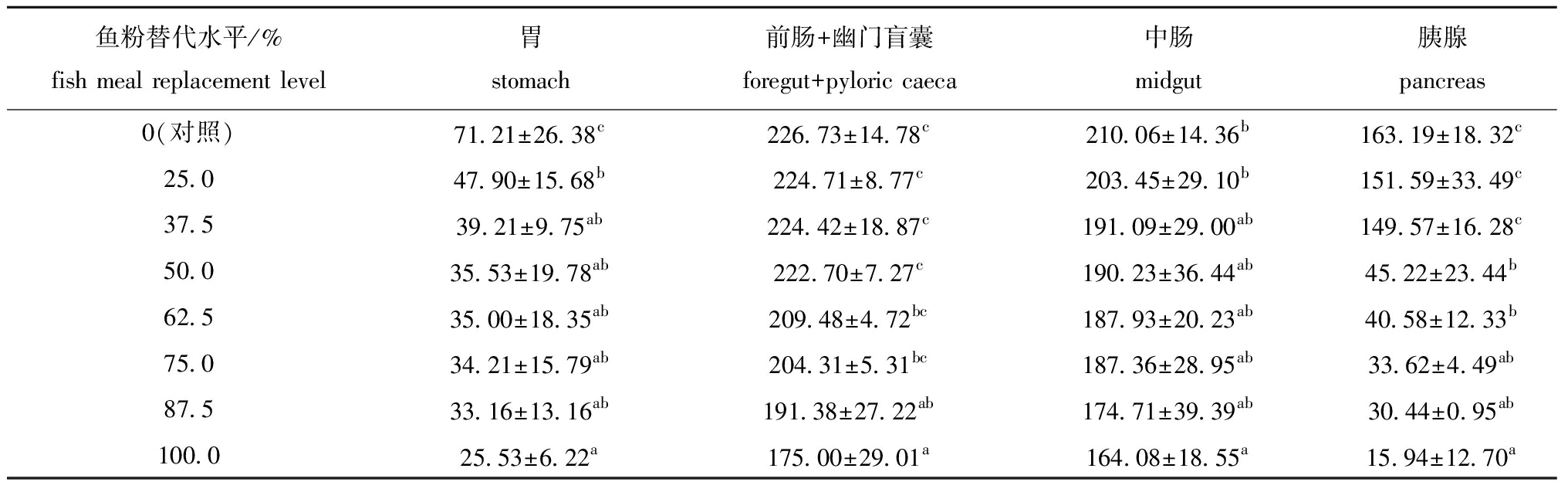
鱼粉替代水平/%fish meal replacement level胃stomach前肠+幽门盲囊foregut+pyloric caeca中肠midgut胰腺pancreas0(对照)71.21±26.38c226.73±14.78c210.06±14.36b163.19±18.32c25.047.90±15.68b224.71±8.77c203.45±29.10b151.59±33.49c37.539.21±9.75ab224.42±18.87c191.09±29.00ab149.57±16.28c50.035.53±19.78ab222.70±7.27c190.23±36.44ab45.22±23.44b62.535.00±18.35ab209.48±4.72bc187.93±20.23ab40.58±12.33b75.034.21±15.79ab204.31±5.31bc187.36±28.95ab33.62±4.49ab87.533.16±13.16ab191.38±27.22ab174.71±39.39ab30.44±0.95ab100.025.53±6.22a175.00±29.01a164.08±18.55a15.94±12.70a
表5大豆分离蛋白替代鱼粉对哲罗鱼肠道形态的影响
Tab.5EffectsoffishmealreplacementbysoybeanproteinisolateonintestinalmorphologyoftaimenHuchotaimen
μm

鱼粉替代水平/%fish meal replacement level绒毛高度 height of villus纹状缘高度 height of microvillus0(对照)25.037.550.062.575.087.5100.0225.82±4.51d222.07±8.83d206.29±5.17c206.96±1.86c200.40±0.59abc203.17±5.43bc194.21±2.61a195.01±2.62ab4.85±0.38d4.46±0.67cd3.78±0.81bc3.39±0.16ab3.06±0.08ab3.01±0.12a2.79±0.15a2.80±0.13a
3讨论
3.1大豆分离蛋白替代鱼粉对哲罗鱼消化酶活性的影响
饲料中以大豆蛋白为主要蛋白源时,其含有的抗营养因子影响了鱼体对蛋白质的吸收和利用。本研究表明,随着大豆分离蛋白替代水平的增加,哲罗鱼胃、前肠、幽门盲囊、中肠和胰腺的蛋白酶活性下降。对虹鳟Oncorhynchusmykiss[19]、大西洋鲑Salmosalar[20]等的研究中,也表现出类似的变化规律。大豆蛋白影响鱼体蛋白酶活性的机理是抗营养因子(如胰蛋白酶抑制因子)影响胰腺的发育,蛋白酶的合成与分泌量减少,或者其与蛋白酶结合使肠道蛋白酶活性降低[20]。
饲料中高比例大豆产品替代鱼粉后,舌齿鲈Dicentrarchuslabrax[21]、珍珠龙胆石斑鱼(Epinephelusfuscoguttatus♀×E.lanceolatus♂)[22]等肠道的脂肪酶和淀粉酶活性降低,与本试验结果类似。刘文斌等[23]认为,这可能是肠道内部小肽和游离氨基酸的含量增加,消化酶所作用的底物减少,从而降低了内源酶的需要量,此反馈调节了消化酶的表达量。有些鱼类对大豆蛋白的适应性反应有所不同。如翘嘴红鲌Erythroculterilishaeformis[24]肠道蛋白酶活性随着大豆蛋白替代鱼粉比例的增加而降低,肠道脂肪酶和淀粉酶未出现明显变化。埃及胡子鲇Clariasleather肠道脂肪酶亦未发生明显变化[25]。这些结果的差异,不仅与鱼的种类、食性有关,还与大豆产品的种类等试验条件不同有关。
3.2大豆分离蛋白替代鱼粉对哲罗鱼消化系统组织结构的影响
饲料中添加高比例的大豆蛋白后,鱼类肠道的组织形态发生了变化。豆粕替代鱼粉后,埃及胡子鲇肠道绒毛高度有所下降[25];大豆分离蛋白替代鱼粉也会引起幼建鲤Cyprinuscarpiovar. Jian肠绒毛高度降低,消化吸收能力减弱[26]。本研究结果显示,高比例(替代水平>75%)的大豆蛋白替代鱼粉后,肠道的绒毛和微绒毛高度显著下降,这可能与大豆蛋白中的抗原分子抑制肠细胞的生长发育有关。
鱼类肠道结构受损后,会发生黏膜炎症反应,消化吸收能力下降,生长性能降低。饲料中高比例大豆蛋白替代鱼粉后,大西洋鲑[27-28]、虹鳟[29]、大西洋鲽Hippoglossushippoglossus[30]等肠道上皮完整性和正常形态结构受到破坏后,会发生肠道上皮脱落、结缔组织数量增加、黏膜固有层和黏膜下层细胞核排列紊乱、炎性细胞(淋巴细胞、巨噬细胞和多核细胞)浸润等。杂食性鱼类鲤摄食豆粕和大豆分离蛋白替代鱼粉饲料时,前肠和后肠的完整性也会受到破坏,出现肠上皮细胞脱落等形态改变[26]。吴莉芳等[31]认为,这可能是上皮细胞表面的特异性受体与大豆中的某些致敏原结合,导致肠黏膜出现炎症反应所致。
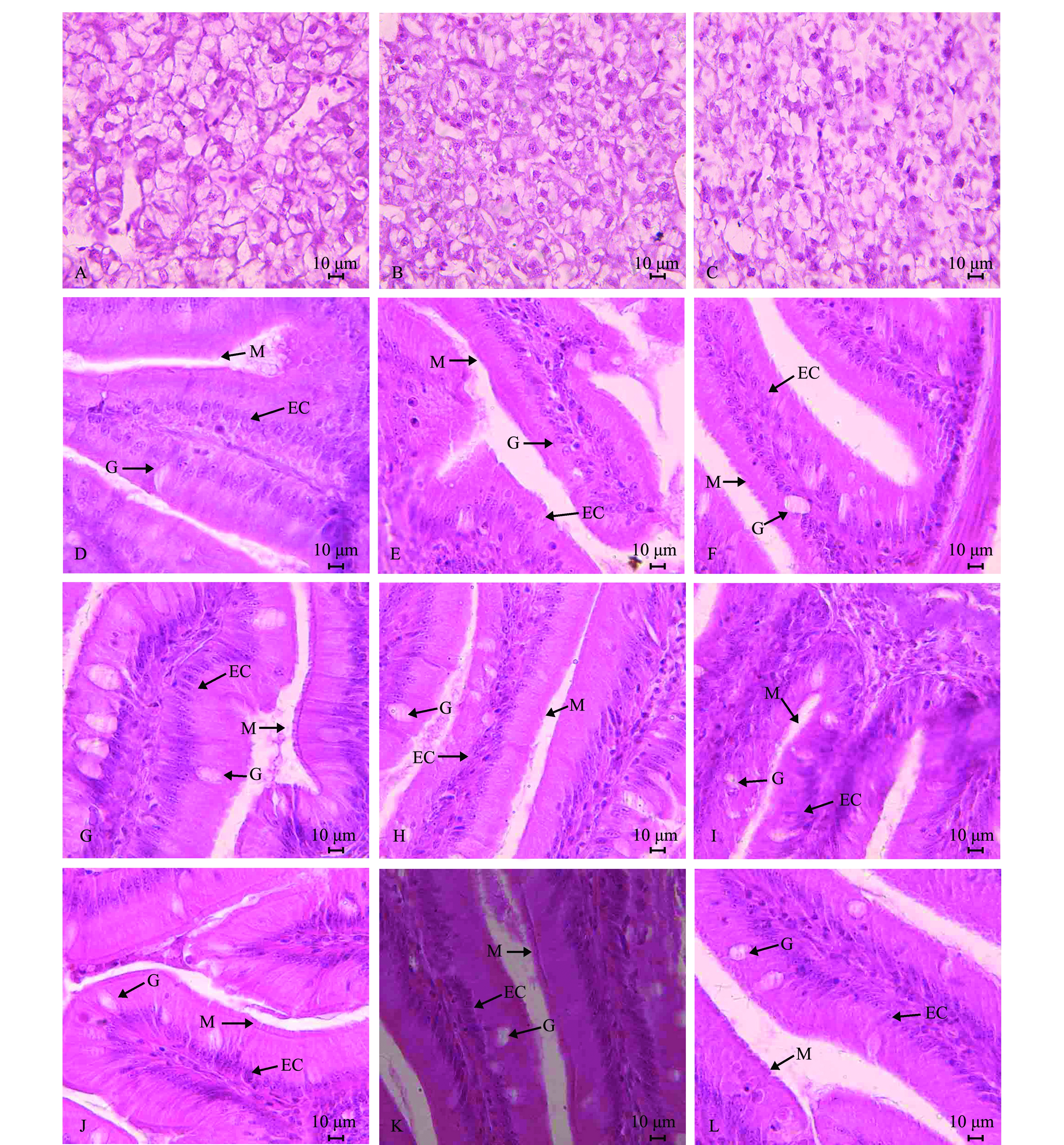
注:A为对照组肝脏;B为75.0%替代组肝脏;C为100.0%替代组肝脏;D为对照组幽门盲囊;E为75.0%替代组幽门盲囊;F为100.0%替代组幽门盲囊;G为对照组前肠;H为75.0%替代组前肠;I为100.0%替代组前肠;J为对照组中肠;K为75.0%替代组中肠;L为100.0%替代组中肠(100×)。M为纹状缘; G为杯状细胞; EC为上皮细胞
Note: A, liver in control group; B, liver in the 75.0% replacement group; C, liver in the 100.0% replacement group; D, pyloric caeca in control group; E, pyloric caeca in the 75.0% replacement group; F, pyloric caeca in the 100.0% replacement group; G, foregut in control group; H, foregut in the 75.0% replacement group; I, foregut in the 100.0% replacement group; J, midgut in control group; K, midgut in the 75.0% replacement group; L, midgut in the 100.0% replacement group (100×). M,Microvilli; G,Goblet cells; EC,Epithelial cells
图1 大豆分离蛋白替代鱼粉对哲罗鱼肠道和肝脏形态的影响
Fig.1 Effects of fish meal replacement by soybean protein isolate on intestinal and hepatic morphology of taimen Hucho taimen
饲料中大豆蛋白高比例替代鱼粉后,大黄鱼Larimichthyscrocea[32]、鲈Lateolabraxjaponicus[33]等肉食性鱼类的肠道组织和肝脏会出现明显的结构性病变。本研究表明,当大豆分离蛋白替代鱼粉至75%时(含鱼粉10%),哲罗鱼肠道绒毛明显稀疏且出现空泡,肝细胞明显减少,且细胞核大量融解,甚至消失。这可能与大豆分离蛋白中的胰蛋白酶抑制因子、凝集素、皂甙等抗营养因子抑制肠道和肝脏细胞的发育有关,肠道和肝脏组织也因此受到破坏。对金头鲷Sparusaurata[34]、大黄鱼[32]等研究也表明,肝发生病变时,还会出现脂肪蓄积。其原因一是脂蛋白合成减少,肝细胞内的脂肪不能及时转运出来,二是大豆蛋白中的毒性物质引起的脂肪代谢失调。
3.3大豆分离蛋白替代鱼粉对哲罗鱼生长抑制的消化生理机制
前期研究表明,与其他肉食性鱼类如南方鲇Silurusmeridionalis[35]等类似,饲料中用大豆分离蛋白替代鱼粉比例增加时,哲罗鱼的生长性能下降,当替代鱼粉的比例超过60%时,其生长率、饲料转化率和蛋白质沉积率极显著下降[17]。这可能是由于大豆蛋白中的抗营养因子阻碍营养物质消化吸收,以及其氨基酸比例不平衡所引起的[36]。从本试验结果来看,大豆蛋白中的抗营养因子抑制了哲罗鱼肝脏、肠道的发育,高比例替代鱼粉(>75.0%)时,甚至导致其结构性病变,引起蛋白酶、脂肪酶和淀粉酶分泌量减少,进而减少了鱼体对营养物质的消化吸收。因此,饲料中采用大豆分离蛋白替代鱼粉的过程中,需要采用适当地去抗营养因子的方法以提高其利用率。
参考文献:
[1] Tacon A G J,Metian M.Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds:trends and future prospects[J].Aquaculture,2008,285(1-4):146-158.
[2] Zhang Chunxiao,Rahimnejad S,Wang Yaru,et al.Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabraxjaponicus):effects on growth,digestive enzymes activity,gut histology,and expression of gut inflammatory and transporter genes[J].Aquaculture,2018,483:173-182.
[3] Aragona M,Lauriano E R,Pergolizzi S,et al.Opuntiaficusindica(L.) Miller as a source of bioactivity compounds for health and nutrition[J].Natural Product Research,2017:1-13,doi:10.1080/14786419.2017.1365073.
[4] Carbone D,Faggio C.Importance of prebiotics in aquaculture as immunostimulants.Effects on immune system ofSparusaurataandDicentrarchuslabrax[J].Fish & Shellfish Immunology,2016,54:172-178.
[5] Burgos-Aceves M A,Cohen A,Smith Y,et al.Estrogen regulation of gene expression in the teleost fish immune system[J].Fish & Shellfish Immunology,2016,58:42-49.
[6] GatlinIII D M,Barrows F T,Brown P,et al.Expanding the utilization of sustainable plant products in aquafeeds:a review[J].Aquaculture Research,2007,38(6):551-579.
[7] Francis G,Makkar H P S,Becker K.Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish[J].Aquaculture,2001,199(3-4):197-227.
[8] Zhou Q C,Mai K S,Tan B P,et al.Partial replacement of fishmeal by soybean meal in diets for juvenile cobia (Rachycentroncanadum)[J].Aquaculture Nutrition,2005,11(3):175-182.
[9] Hedrera M I,Galdames J A,Jimenez-Reyes M F,et al.Soybean meal induces intestinal inflammation in zebrafish larvae[J].PLoS One,2013,8(7):e69983.
[10] Barrows F T,Stone D A J,Hardy R W.The effects of extrusion conditions on the nutritional value of soybean meal for rainbow trout (Oncorhynchusmykiss)[J].Aquaculture,2007,265(1-4):244-252.
[11] Carter C G,Hauler R C.Fish meal replacement by plant meals in extruded feeds for Atlantic salmon,SalmosalarL.[J].Aquaculture,2000,185(3-4):299-311.
[12] Bakke-McKellep A M,Frøystad M K,Lilleeng E,et al.Response to soy:T-cell-like reactivity in the intestine of Atlantic salmon,SalmosalarL.[J].Journal of Fish Diseases,2007,30(1):13-25.
[13] Kikuchi K.Use of defatted soybean meal as a substitute for fish meal in diets of Japanese flounder (Paralichthysolivaceus)[J].Aquaculture,1999,179(1-4):3-11.
[14] 徐奇友,王炳谦,徐连伟,等.哲罗鱼稚鱼的蛋白质和脂肪需求量[J].中国水产科学,2007,14(3):498-503.
[15] 张美彦,王常安,徐奇友.肌醇对哲罗鲑生长性能、体成分及消化酶活性的影响[J].中国水产科学,2014,21(3):560-566.
[16] Wang Chang’an,Li Jinnan,Wang Liansheng,et al.Effects of dietary phosphorus on growth,body composition and immunity of young taimenHuchotaimen(Pallas,1773)[J].Aquaculture Research,2017,48(6):3066-3079.
[17] 徐奇友,王常安,许红,等.大豆分离蛋白替代鱼粉对哲罗鱼稚鱼生长、体成分和血液生化指标的影响[J].水生生物学报,2008,32(6):941-946.
[18] 桂远明.水产动物机能学实验[M].北京:中国农业出版社,2004:92-93.
[19] Krogdahl Å,Lea T B,Olli J J.Soybean proteinase inhibitors affect intestinal trypsin activities and amino acid digestibilities in rainbow trout (Oncorhynchusmykiss)[J].Comparative Biochemistry and Physiology Part A:Physiology,1994,107(1):215-219.
[20] Sveier H,Kvamme B O,Raae A J.Growth and protein utilization in Atlantic salmon (SalmosalarL.) given a protease inhibitor in the diet[J].Aquaculture Nutrition,2001,7(4):255-264.
[21] Cahu C L,Infante J L Z,Quazuguel P,et al.Protein hydrolysate vs.fish meal in compound diets for 10-day old sea bassDicentrarchuslabraxlarvae[J].Aquaculture,1999,171(1-2):109-119.
[22] 李学丽,王际英,宋志东,等.酶解豆粕替代鱼粉对珍珠龙胆石斑鱼幼鱼生长和主要代谢酶活力的影响[J].海洋渔业,2017,39(5):529-538.
[23] 刘文斌,王恬.棉粕蛋白酶解物对异育银鲫(Carassiusauratusgibelio)消化、生长和胰蛋白酶mRNA表达量的影响[J].海洋与湖沼,2006,37(6):568-574.
[24] 钱曦,王桂芹,周洪琪,等.饲料蛋白水平及豆粕替代鱼粉比例对翘嘴红鲌消化酶活性的影响[J].动物营养学报,2007,19(2):182-187.
[25] 吴莉芳,秦贵信,孙泽威,等.饲料中去皮豆粕替代鱼粉对埃及胡子鲇消化酶活力和肠道组织的影响[J].中山大学学报:自然科学版,2010,49(4):99-105.
[26] 张锦秀.大豆蛋白源对幼建鲤生长性能及肠道免疫的影响[D].雅安:四川农业大学,2003.
[27] Refstie S,Storebakken T,Baeverfjord G,et al.Long-term protein and lipid growth of Atlantic salmon (Salmosalar) fed diets with partial replacement of fish meal by soy protein products at medium or high lipid level[J].Aquaculture,2001,193(1-2):91-106.
[28] Krogdahl Å,Bakke-McKellep A M,Baeverfjord G.Effects of graded levels of standard soybean meal on intestinal structure,mucosal enzyme activities,and pancreatic response in Atlantic salmon (SalmosalarL.)[J].Aquaculture Nutrition,2003,9(6):361-371.
[29] Heikkinen J,Vielma J,Kemiläinen O,et al.Effects of soybean meal based diet on growth performance,gut histopathology and intestinal microbiota of juvenile rainbow trout(Oncorhynchusmykiss)[J].Aquaculture,2006,261(1):259-268.
[30] Murray H M,Lall S P,Rajaselvam R,et al.A nutrigenomic analysis of intestinal response to partial soybean meal replacement in diets for juvenile Atlantic halibut,Hippoglossushippoglossus, L.[J].Aquaculture,2010,298(3-4):282-293.
[31] 吴莉芳,瞿子惠,周锴,等.豆粕替代鱼粉对黄金鲈生长及肠道组织的影响[J].西北农林科技大学学报:自然科学版,2017,45(6):1-8.
[32] 冯建,王萍,何娇娇,等.大豆浓缩蛋白替代鱼粉对大黄鱼幼鱼生长、体成分、血清生化指标及肝组织学的影响[J].中国水产科学,2017,24(2):268-277.
[33] Hu Liang,Yun Biao,Xue Min,et al.Effects of fish meal quality and fish meal substitution by animal protein blend on growth performance,flesh quality and liver histology of Japanese seabass (Lateolabraxjaponicus)[J].Aquaculture,2013,372-375:52-61.
[34] Martínez-Llorens S,Baeza-Ari o R,Nogales-Mérida S,et al.Carob seed germ meal as a partial substitute in gilthead sea bream (Sparusaurata) diets:amino acid retention,digestibility,gut and liver histology[J].Aquaculture,2012,338-341:124-133.
o R,Nogales-Mérida S,et al.Carob seed germ meal as a partial substitute in gilthead sea bream (Sparusaurata) diets:amino acid retention,digestibility,gut and liver histology[J].Aquaculture,2012,338-341:124-133.
[35] 艾庆辉,谢小军.南方鲇的营养学研究:饲料中大豆蛋白水平对生长的影响[J].水生生物学报,2002,26(1):57-65.
[36] Hardy R W.Alternate protein sources for salmon and trout diets[J].Animal Feed Science and Technology,1996,59(1-3):71-80.
EffectsoffishmealreplacementbysoybeanproteinisolateondigestivephysiologyoftaimenHuchotaimen
WANG Chang-an, LIU Hong-bai, XU Qi-you, LI Jin-nan, WANG Lian-sheng, ZHAO Zhi-gang, YIN Jia-sheng
(Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Harbin 150070,China)
Abstract:TaimenHuchotaimenwith initial body weight of (6.90±0.04)g were reared in a 220 L indoor flowing glass fiber reinforced plastic tank and fed diets in which fish meal was replaced by 0, 25.0%, 37.5%, 50.0%, 62.5%, 75.0%, 87.5% and 100.0% of soy protein isolate(SPI), diet containing 40.0% fish meal as control, with triplicates of each 100 fish at water temperature of 9.8-16.2 ℃ for 56 d to evaluate the effects of replacing fish meal with SPI on the activities of digestive enzymes and histological structure of digestive tract of taimen. It was found that protease, lipase and amylase activities in stomach, foregut, midgut, pyloric caeca and pancreas were significantly decreased with increasing SPI content (P<0.05). Heights of intestinal villi and microvilli were also shown to be significant decrease (P<0.05), and damaged intestinal structural integrity, fused microvilli, even abscission, and more hepatic vacuoles were observed in the taimen fed high dietary levels of SPI (87.5% and 100.0%) under a microscope, even with blurred boundaries, and shifted and dissolved or vanished nuclei in hepatic cells. It is concluded that fish meal replacement by high level of SPI leads to decline the digestive enzyme activity and to cause loss of function of liver and intestine.
Keywords:Huchotaimen; soybean protein isolate; digestive enzyme; histological structure
通信作者:刘红柏(1970—),女,博士,研究员。E-mail:liuhongbai@hrfri.ac.cn 徐奇友(1969—),男,博士,研究员。E-mail:xuqiyou@hrfri.ac.cn
作者简介:王常安(1981—),男,博士,副研究员。E-mail:gordoncase@126.com
基金项目:国家现代农业产业技术体系专项(CARS-46);黑龙江省自然科学基金资助项目(QC2015041);黑龙江水产研究所基本科研业务费资助项目(HSY201512)
收稿日期:2017-12-11
文章编号:2095-1388(2018)05-0607-07
DOI:10.16535/j.cnki.dlhyxb.2018.05.010
文献标志码:A
中图分类号:S963.73






 o R,Nogales-Mérida S,et al.Carob seed germ meal as a partial substitute in gilthead sea bream (
o R,Nogales-Mérida S,et al.Carob seed germ meal as a partial substitute in gilthead sea bream (