饲料中添加混合益生菌对刺参幼参免疫反应和抗氧化性能的影响
李璐瑶1,孙丕海2,包鹏云1,张丛尧1,杨志平3,马悦欣1
(1.大连海洋大学农业部北方海水增养殖重点实验室,辽宁 大连116023;2.大连海洋大学 海珍品苗种培育基地,辽宁 大连116023;3.大连汇新钛设备开发有限公司,辽宁大连116039)
摘要:为探讨混合益生菌对刺参Apostichopus japonicus幼参免疫反应和抗氧化性能的影响,用添加梅奇酵母C14(浓度为1×105cells/g饲料)、红酵母H26(浓度为1×105cells/g饲料)和芽孢杆菌BC26(浓度为1×107cells/g饲料)的混合益生菌饲料,投喂体质量为(0.54 g±0.06 g)的幼参,记为益生菌组,并设只投喂基础饲料的对照组,投喂4周和8周后,测定幼参免疫指标和抗氧化指标的变化。结果表明:饲养4周时,益生菌组幼参体腔细胞吞噬活力和呼吸爆发,体腔液 (CF)和体腔细胞裂解液 (CLS)溶菌酶、酚氧化酶活力均显著高于对照组 (P<0.05);饲养8周时,益生菌组除CF和CLS酚氧化酶活力与对照组无显著性差异外(P>0.05),其他免疫指标仍显著高于对照组(P<0.05);饲养4周时,益生菌组幼参CF、肠道和体壁超氧化物歧化酶(SOD)活力,CF、CLS、肠道、呼吸树和体壁过氧化氢酶(CAT)活力,总抗氧化能力(TAOC),以及肠道和呼吸树谷胱甘肽(GSH)含量均显著高于对照组(P<0.05);饲养8周时,益生菌组幼参肠道、呼吸树和体壁SOD活力,所有组织CAT活力,CLS和肠道T-AOC,CF、肠道和呼吸树GSH含量均显著高于对照组(P<0.05)。研究表明,饲料中补充混合益生菌可促进幼参免疫反应和影响其抗氧化性能。
关键词:刺参;益生菌;免疫反应;抗氧化性能
刺参Apostichopus japonicus是中国北方地区重要的海珍品养殖种类。但多种传染性疾病给刺参养殖业造成了巨大的经济损失,限制了该产业的可持续发展[1-3]。近年来,益生菌在刺参养殖业中的应用受到一些学者的关注。将乳酸菌L-2和芽孢杆菌Bacillus sp.K-3混合添加到饲料中养殖幼参,可提高幼参肠道超氧化物歧化酶 (SOD)活力[4];枯草芽孢杆菌Bacillus subtilis T13和芽孢杆菌Bacillus sp.BC26能促进幼参体腔细胞吞噬和/或呼吸爆发[5-6];马氏副球菌Paracoccus marcusii DB11可提高幼参体腔液 (含细胞)、呼吸树SOD和过氧化氢酶 (CAT)活力[7];假交替单胞菌Pseudoalteromonas elyakovii HS1、希瓦氏菌Shewanella japonica HS7和塔斯马尼亚弧菌Vibrio tasmaniensis HS10可增强幼参体腔细胞呼吸爆发和吞噬活力,以及体腔细胞裂解液 (CLS)溶菌酶和SOD活力[8];梅奇酵母Metschnikowia sp.C14和仙人掌有孢汉逊酵母Hanseniaspora opuntiae C21可刺激幼参体腔细胞吞噬活力,以及体腔液 (CF)溶菌酶、酚氧化酶、CLS溶菌酶和 SOD 活力[9-10]; 海洋红酵母Rhodotorula benthica D30可显著增加幼参体腔细胞的吞噬活力,以及CF的溶菌酶、酚氧化酶和SOD活力[11]。但混合酵母菌和细菌对幼参免疫反应和抗氧化性能的影响目前尚未见报道,本研究中,分析了由梅奇酵母C14、红酵母 H26和芽孢杆菌BC26[6,12]组成的混合菌对幼参免疫指标和抗氧化性能的影响,旨在为混合益生菌在刺参养殖中的应用提供参考。
1 材料与方法
1.1 材料
试验用健康刺参幼参购自大连市某刺参养殖场。基础饲料配方同文献[9]。
1.2 方法
1.2.1 含菌饲料的制备 将梅奇酵母C14菌株和红酵母H26菌株接种于酵母膏胨葡萄糖 (YPD)液体培养基中,将芽孢杆菌BC26菌株接种于胰蛋白胨大豆肉汤 (TSB)培养基中,在25℃下震荡培养16 h,将菌悬液离心,用生理盐水重悬细胞,并用血球计数板计数,然后添加到幼参基础饲料中,制备成含C14菌株 (1×105cells/g饲料)+ H26菌株 (1×105cells/g饲料)+BC26菌株 (1× 107cells/g饲料)的饲料,菌株剂量依据文献[6,9]确定,饲料每天制备一次以保证菌株活力。
1.2.2 试验设计 将幼参暂养2周后,选择大小相近的幼参(0.54 g±0.06 g)随机分配到6个盛有过滤海水的塑料桶中,每桶200头,用含菌饲料和基础饲料分别投喂3桶幼参,分别记为益生菌组和对照组。每日投喂一次,投喂量为其体质量的5%,每2 d换水1/2,吸底,用气石充气。试验期间,温度为16~24℃,pH为7.8~8.2,盐度为30。
1.2.3 样品的采集 养殖4周和8周时,分别从每个平行随机取10头幼参,饥饿20 h,使肠道内容物排空。用无菌海水冲洗幼参体表,断尾解剖,取其体腔液500 μL加入等体积抗凝剂[13],混合均匀,取400 μL进行体腔细胞计数、呼吸爆发和吞噬活力测定,剩余体腔液在4℃下离心,取上清液即CF;沉淀用体腔细胞等渗液[13]重悬,将重悬液在冰浴中用超声波破碎仪破碎,在4℃下离心,上清液即CLS,测定CF和CLS的免疫指标和抗氧化指标。
取肠道、呼吸树和体壁组织迅速用液氮冷冻后于超低温冰箱 (-80℃)中保存;将各组织匀浆液在4℃下离心,取上清液测定其抗氧化指标。
1.2.4 蛋白含量的测定 采用考马斯亮蓝法[14]测定CF、CLS、肠道、呼吸树和体壁匀浆液上清液中总蛋白含量。
1.2.5 免疫指标的检测 体腔细胞吞噬活力的测定依据Ma等[10]的方法进行,以50 μL样品中每毫克蛋白吞噬的酵母细胞数量表示吞噬活力。参考Song等[15]的方法略加修改,进行体腔细胞呼吸爆发的测定。具体如下:于1.5 mL离心管中加入100 μL 0.1%多聚赖氨酸,然后加入100 μL抗凝体腔液,离心,去上清液,再加入100 μL佛波醇PMA,室温 (20~24℃)下孵育30 min;加入100 μL 0.3%氯化硝基四氮唑蓝NBT,室温下孵育30 min,孵育结束后于4℃下离心10 min,去上清液,加入200 μL甲醇中止反应10 min,4℃下离心10 min,去上清液,用70%甲醇洗涤,室温下晾干;加入120 μL KOH和140 μL二甲基亚砜DMSO,充分溶解后将液体加入酶标板,利用Epoch酶标仪(美国BioTek公司)测定630 nm波长处的吸光值,呼吸爆发以每106cells的OD值表示。
溶菌酶活力的测定以溶壁微球菌冻干粉为底物,依据Hultmark等[16]的方法进行并加以改进。用0.1 mol/L pH 6.4的磷酸盐缓冲液配制成底物(OD570 nm=0.2~0.3),取300 μL该悬液加入5 μL CF或CLS,对照以等体积PBS代替样品。混合后于室温 (20~24℃)下孵育30 min,冰浴1 h,测定570 nm处的吸光值,对照记为A0,样品记为A,溶菌酶活力=(A0-A)/A。
酚氧化酶活力的测定参考文献 [6,17]的方法进行。酚氧化酶活力单位定义为:在试验条件下,每分钟 OD490 nm值增加0.001为一个酶活力单位 (U)。
1.2.6 抗氧化指标的检测 CF、CLS、肠道、呼吸树和体壁的 SOD、CAT活力、总抗氧化能力(T-AOC)和还原型谷胱甘肽 (GSH)含量均采用试剂盒 (购于南京建成科技有限公司)检测,利用Epoch酶标仪进行测定,检测方法参照说明书(将反应温度37℃修改为室温20~24℃)。
1.3 数据处理
试验数据采用平均值±标准差 (mean±S.D.)表示,用SPSS 16.0软件进行独立样本t检验,置信度设为95%。
2 结果与分析
2.1 益生菌对刺参幼参免疫指标的影响
从图1-A、B可见:饲养4周和8周时,益生菌组幼参体腔细胞吞噬活力和呼吸爆发均显著高于对照组 (P<0.05);对照组和益生菌组体腔细胞吞噬活力在4周时均显著高于8周 (P<0.05),益生菌组体腔细胞呼吸爆发在8周时显著高于4周时(P<0.05),而对照组体腔细胞呼吸爆发在4周和8周时无显著性差异 (P>0.05)。
从图1-C、D可见:饲养4周和8周时,益生菌组幼参CF和CLS溶菌酶活力均显著高于对照组(P<0.05);益生菌组CF、CLS溶菌酶活力和对照组CLS溶菌酶活力均在8周时显著高于4周时(P<0.05),而对照组CF溶菌酶活力在4周和8周时无显著性差异 (P>0.05)。
从图1-E、F可见:饲养4周时,益生菌组幼参CF和CLS酚氧化酶活力均显著高于对照组(P<0.05),饲养8周时,益生菌组与对照组的CF 和CLS酚氧化酶活力均无显著性差异 (P>0.05);对照组CF和CLS酚氧化酶活力在8周时显著高于4周时 (P<0.05),而益生菌组CF和CLS酚氧化酶活力在4周和8周时无显著性差异 (P>0.05)。
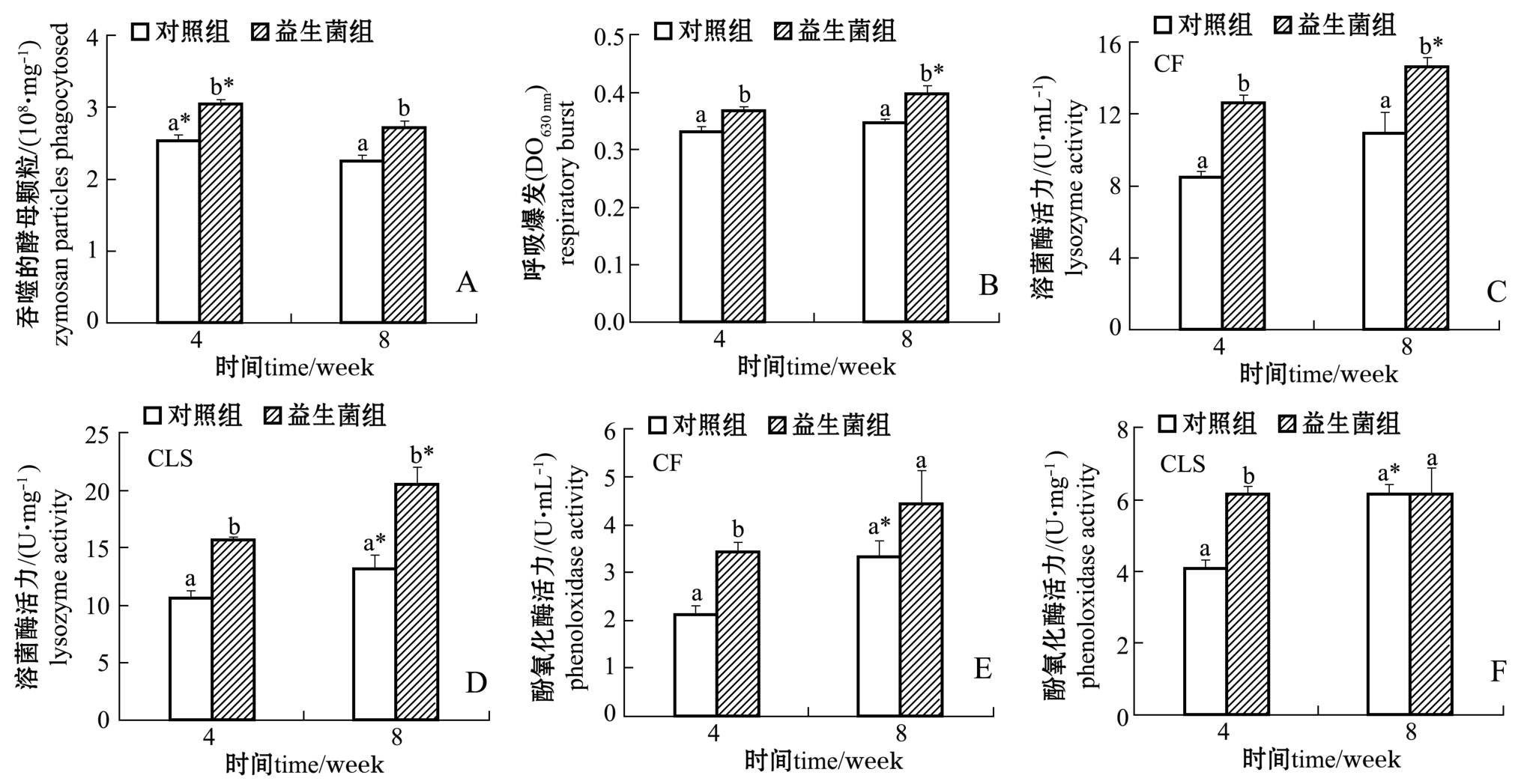
图1 益生菌对幼参体腔细胞吞噬活力和呼吸爆发以及对体腔液和体腔细胞裂解液的溶菌酶、酚氧化酶活力的影响
Fig.1 Effects of probiotics on phagocytic activity and respiratory burst in coelomocytes,and on lysozyme and phenoloxidase activities in coelomic fluid(CF)and coelomocyte cell lysate supernatant(CLS)of juvenile sea cucumber Apostichopus japonicus
注:不同字母表示同一采样时间下不同组间有显著性差异 (P<0.05);*表示同一组在不同采样周组间有显著性差异 (P<0.05)
Note:The means with different letters in different groups in the same week are significant differences at the 0.05 probability level;*means significant differences in the same group in different weeks(P<0.05)
2.2 益生菌对刺参幼参抗氧化指标的影响
从表1可见:饲养4周时,仅益生菌组幼参CF、肠道和体壁SOD活力显著高于对照组 (P<0.05),饲养8周时,仅益生菌组幼参肠道、呼吸树和体壁SOD活力显著高于对照组 (P<0.05),其余组织SOD活力在4周和8周时与对照组均无显著性差异 (P>0.05);对照组幼参CF、CLS和肠道SOD活力在4周时显著高于8周时 (P<0.05),益生菌组CF、肠道和体壁SOD活力在4周时显著高于8周时 (P<0.05),其余组织SOD活力在4周和8周时无显著性差异 (P>0.05)。
从表1可见:饲养4周和8周时,益生菌组幼参CF、CLS、肠道、呼吸树和体壁CAT活力均显著高于对照组 (P<0.05);对照组和益生菌组幼参CF、CLS、肠道和呼吸树CAT活力在8周时显著高于4周时 (P<0.05),但体壁CAT活力在4周时显著高于8周时 (P<0.05)。
从表1可见:饲养4周时,益生菌组幼参CF、 CLS、肠道、呼吸树和体壁T-AOC均显著高于对照组 (P<0.05),饲养8周时,仅益生菌组CLS和肠道T-AOC显著高于对照组 (P<0.05),其余组织T-AOC与对照组均无显著性差异 (P>0.05);对照组幼参 CF、CLS、肠道、呼吸树和体壁 TAOC在8周时显著高于4周时 (P<0.05),而益生菌组仅肠道和体壁T-AOC在8周时显著高于4周时 (P<0.05),其余组织T-AOC在4周和8周时均无显著性差异 (P>0.05)。
从表1可见:饲养4周时,益生菌组幼参仅肠道和呼吸树GSH含量显著高于对照组 (P<0.05),其余组织GSH含量与对照组无显著性差异 (P>0.05),饲养8周时,益生菌组仅CF、肠道和呼吸树GSH含量显著高于对照组 (P<0.05),其余组织GSH含量与对照组无显著性差异 (P>0.05);对照组幼参仅CF、肠道和呼吸树GSH含量在4周时显著高于8周时 (P<0.05),而益生菌组幼参CF的GSH含量在8周时显著高于4周时 (P<0.05),肠道和呼吸树GSH含量在4周时显著高于8周时 (P<0.05),其余组织GSH含量在4周和8周时均无显著性差异 (P>0.05)。
综上所述,饲料中添加混合益生菌可增强幼参体腔细胞吞噬活力、呼吸爆发,以及CF、CLS的溶菌酶活力和酚氧化酶活力;可提高幼参CF、肠道、呼吸树和体壁的SOD活力,以及CF、CLS、肠道、呼吸树、体壁的CAT活力和T-AOC;还可提高CF、肠道和呼吸树的GSH含量;但不影响幼参CLS的SOD活力以及提高CLS和体壁的GSH含量。
表1 益生菌对幼参不同组织SOD、CAT活力、T-AOC和GSH含量的影响
Tab.1 Effects of probiotics on activities of superoxide dismutase(SOD)and catalase(CAT),T-AOC,and glutathione (GSH)content in different tissues of juvenile sea cucumber Apostichopus japonicus U/mg
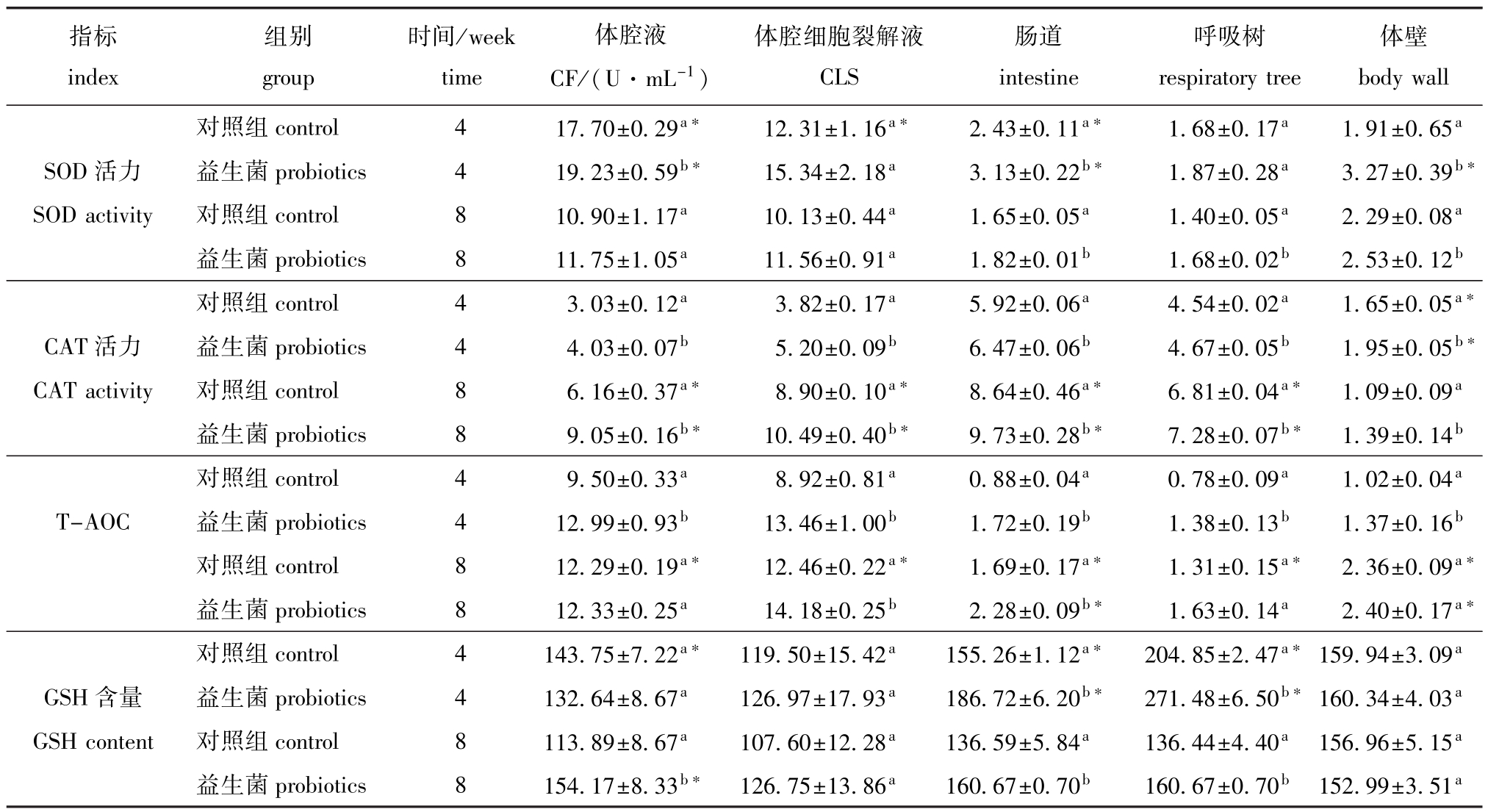
注:不同小写字母表示在同一采样时间下同一组织不同组间有显著性差异 (P<0.05),*表示同一组的同一组织于不同采样周间有显著性差异 (P<0.05)
Note:The means with different letters in the same tissue in different groups at the same week are significant differences at the 0.05 probability level,*means significant differences in the same tissue in the same group at different weeks(P<0.05)
指标index时间/week time组别group体腔液CF/(U·mL-1)体腔细胞裂解液CLS肠道intestine呼吸树respiratory tree体壁body wall对照组control 4 17.70±0.29a* 12.31±1.16a* 2.43±0.11a* 1.68±0.17a 1.91±0.65aSOD活力 益生菌probiotics 4 19.23±0.59b* 15.34±2.18a 3.13±0.22b* 1.87±0.28a 3.27±0.39b*SOD activity 对照组control 8 10.90±1.17a 10.13±0.44a 1.65±0.05a 1.40±0.05a 2.29±0.08a益生菌probiotics 8 11.75±1.05a 11.56±0.91a 1.82±0.01b 1.68±0.02b 2.53±0.12b对照组control 4 3.03±0.12a 3.82±0.17a 5.92±0.06a 4.54±0.02a 1.65±0.05a*CAT活力 益生菌probiotics 4 4.03±0.07b 5.20±0.09b 6.47±0.06b 4.67±0.05b 1.95±0.05b*CAT activity 对照组control 8 6.16±0.37a* 8.90±0.10a* 8.64±0.46a* 6.81±0.04a* 1.09±0.09a益生菌probiotics 8 9.05±0.16b* 10.49±0.40b* 9.73±0.28b* 7.28±0.07b* 1.39±0.14b对照组control 4 9.50±0.33a 8.92±0.81a 0.88±0.04a 0.78±0.09a 1.02±0.04aT-AOC 益生菌probiotics 4 12.99±0.93b 13.46±1.00b 1.72±0.19b 1.38±0.13b 1.37±0.16b对照组control 8 12.29±0.19a* 12.46±0.22a* 1.69±0.17a* 1.31±0.15a* 2.36±0.09a*益生菌probiotics 8 12.33±0.25a 14.18±0.25b 2.28±0.09b* 1.63±0.14a 2.40±0.17a*对照组control 4 143.75±7.22a* 119.50±15.42a 155.26±1.12a* 204.85±2.47a*159.94±3.09aGSH含量 益生菌probiotics 4 132.64±8.67a 126.97±17.93a 186.72±6.20b* 271.48±6.50b*160.34±4.03aGSH content 对照组control 8 113.89±8.67a 107.60±12.28a 136.59±5.84a 136.44±4.40a 156.96±5.15a益生菌probiotics 8 154.17±8.33b* 126.75±13.86a 160.67±0.70b 160.67±0.70b 152.99±3.51a
3 讨论
刺参非特异性免疫防御系统主要包括细胞免疫系统和体液免疫系统,单一菌株酵母菌和细菌可作为幼参免疫增强剂,刺激机体免疫反应[5-7,9-11]。
体腔细胞是棘皮动物抵御感染和损伤的第一道防线,起着吞噬、诱捕和包裹入侵微生物的作用[6,18]。与Macey等[19]报道的混合菌 (2株酵母菌+1株细菌)对南非鲍Haliotis midae血细胞吞噬活力的影响结果类似,本研究中用添加混合菌(梅奇酵母C14、红酵母H26和芽孢杆菌BC26)的饲料投喂幼参,可使幼参体腔细胞的吞噬活力显著增强。溶菌酶作为特异性免疫分子抵抗细菌病原侵入的作用机制,主要是催化细菌尤其是G+菌细胞壁中肽聚糖组分N-乙酰葡萄糖胺和N-乙酰胞壁酸之间β-1,4糖苷键的水解[20]。溶菌酶除了抵抗细菌病原侵入作用外,还可直接促进吞噬作用或作为调理素,这可能与混合菌增强吞噬作用有关联[21]。本研究中,投喂混合益生菌饲料可显著增加幼参CF和CLS的溶菌酶活力。酚氧化酶系统是幼参免疫防御的重要组成部分[6,9-10,22]。本研究中,给幼参投喂混合益生菌4周时其CF和CLS的酚氧化酶活力显著提高。前期研究表明,口服β-葡聚糖可以增强幼参体腔细胞的吞噬活力和CLS酚氧化酶活力[22],肽聚糖能激活斑节对虾Penaeus japonicus粒细胞的吞噬作用[23],推测本研究中酵母菌和细菌的作用机理可能是通过菌体细胞壁成分刺激幼参非特异性免疫反应。
活性氧 (ROS)伴随吞噬作用中的呼吸爆发过程,由细胞膜上的NAD(P)H-氧化酶催化分子氧的单价还原产生。产生的ROS包括超氧阴离子(O-2),如过氧化氢和羟基自由基等,这些代谢产物对广泛大分子的高反应性,使它们成为非常有效的杀菌剂和细胞毒性剂[24-25]。用添加枯草芽孢杆菌T13的饲料投喂幼参,可提高其体腔细胞的呼吸爆发[5]。本研究表明,投喂混合益生菌饲料可以显著提高幼参体腔细胞的呼吸爆发。众所周知,如果ROS过多积累对生物体可能是非常有害的,而SOD和CAT是海参体腔细胞和组织中参与ROS脱毒的抗氧化酶[7,25]。SOD可以催化超氧自由基生成H2O2和O2;CAT可以催化过氧化氢分解成H2O 和O2,从而清除细胞代谢产生的H2O2;T-AOC代表体内酶性和非酶性抗氧化物的总体水平,是反映机体总抗氧化系统功能状态的综合性指标;GSH是水生无脊椎动物体内重要的清除自由基的非酶性抗氧化物[26]。目前,关于投喂益生菌对刺参抗氧化能力影响的报道较少[7]。本研究中,混合益生菌可提高幼参肠道、呼吸树和体壁SOD的活力,提高CF、CLS、肠道、呼吸树和体壁的CAT活力和T-AOC,增加肠道和呼吸树的GSH含量。海洋红酵母D30能提高幼参CF的SOD活力[11];Rhodosporidium paludigenum KGO2可使凡纳滨对虾Litopenaeus vannamei肝胰腺和血清SOD活力,血清和肌肉CAT活力,以及T-AOC显著增加[27];枯草芽孢杆菌能提高凡纳滨对虾血清SOD活力和TAOC[28];海洋酵母能积累虾青素和类胡萝卜素[29-30],摄食添加适量β-胡萝卜素和虾青素饲料的刺参,其体腔液中的T-AOC均显著高于对照组[31]。由此推测,本研究中混合益生菌影响幼参的抗氧化性能可能与红酵母含类胡萝卜素有关,其具体作用机理有待进一步研究。
参考文献:
[1]马悦欣,徐高蓉,张恩鹏,等.仿刺参幼参急性口围肿胀症的细菌性病原[J].水产学报,2006,30(3):377-382.
[2]Deng Huan,He Chongbo,Zhou Zunchun,et al.Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus[J].Aquaculture,2009,287(1-2):18-27.
[3]Li Hua,Qiao Guo,Li Qiang,et al.Biological characteristics and pathogenicity of a highly pathogenic Shewanella marisflavi infecting sea cucumber,Apostichopus japonicus[J].Journal of Fish Disease,2010,33(11):865-877.
[4]张涛,白岚,李蕾,等.不同添加量的益生菌组合对仿刺参消化和免疫指标的影响[J].大连水产学院学报,2009,24(S):64-68.
[5]Zhao Yancui,Zhang Wenbing,Xu Wei,et al.Effects of potential probiotic Bacillus subtilis T13 on growth,immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus[J].Fish&Shellfish Immunology,2012,32(5):750-755.
[6]刘姣,韩华,孙飞雪,等.饵料中添加芽孢杆菌BC26对刺参幼参消化酶、免疫反应和抗病力的影响[J].大连海洋大学学报,2013,28(6):568-572.
[7]Yan Fajun,Tian Xiangli,Dong Shuanglin,et al.Growth performance,immune response,and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus fed a supplementary diet of the potential probiotic Paracoccus marcusii DB11[J].Aquaculture,2014,420-421:105-111.
[8]Chi Cheng,Liu Jiayan,Fei Shizhou,et al.Effect of intestinal autochthonous probiotics isolated from the gut of sea cucumber (Apostichopus japonicus)on immune response and growth of A.japonicus[J].Fish&Shellfish Immunology,2014,38(2):367-373.
[9]Liu Zhiming,Ma Yuexin,Yang Zhiping,et al.Immune responses and disease resistance of the juvenile sea cucumber Apostichopus japonicus induced by Metschnikowia sp.C14[J].Aquaculture,2012,368-369:10-18.
[10]Ma Yuexin,Liu Zhiming,Yang Zhiping,et al.Effects of dietary live yeast Hanseniaspora opuntiae C21 on the immune and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus[J].Fish&Shellfish Immunology,2013,34(1):66-73.
[11]Wang Jihui,Zhao Liuqun,Liu Jinfeng,et al.Effect of potential probiotic Rhodotorula benthica D30 on the growth performance,digestive enzyme activity and immunity in juvenile sea cucumber Apostichopus japonicus[J].Fish&Shellfish Immunology,2015,43(2):330-336.
[12]李明,马悦欣,刘志明,等.刺参机体酵母菌组成及其拮抗活性的研究[J].大连海洋大学学报,2012,27(5):436-440.
[13]Xing Jun,Leung M F,Chia F S.Quantitative analysis of phagocytosis by amebocytes of a sea cucumber,Holothuria leucospilota [J].Invertebrate Biology,1998,117(1):67-74.
[14]Bradford M M.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding[J].Analytical Biochemstry,1976,72(1-2):248-254.
[15]Song Y L,Hsieh Y T.Immunostimulation of tiger shrimp(Penaeus monodon)hemocytes for generation of microbicidal substances:analysis of reactive oxygen species[J].Developmental& Comparative Immunology,1994,18(3):201-209.
[16]Hultmark D,Steiner H,Rasmuson T,et al.Insect immunity.Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia[J]. European Journal of Biochemistry,1980,106(1):7-16.
[17]Smith V J,Söderhäll K.A comparison of phenoloxidase activity in the blood of marine invertebrates[J].Developmental&Comparative Immunology,1991,15(4):251-261.
[18]Gliński Z,Jarosz J.Immune phenomena in echinoderms[J].Archivum Immunologiae et Therapiae Experimentalis,2000,48(3):189-193.
[19]Macey B M,Coyne V E.Improved growth rate and disease resistance in farmed Haliotis midae through probiotic treatment[J]. Aquaculture,2005,245(1-4):249-261.
[20]Jollès P,Jollès J.What's new in lysozyme research?Always amodel system,today as yesterday[J].Molecular and Cellular Biochemistry,1984,63(2):165-189.
[21]Saurabh S,Sahoo P K.Lysozyme:an important defence molecule of fish innate immune system[J].Aquaculture Research,2008,39(3):223-239.
[22]Zhao Yancui,Ma Hongming,Zhang Wenbing,et al.Effects of dietary β-glucan on the growth,immune responses and resistance of sea cucumber,Apostichopus japonicus against Vibrio splendidus infection[J].Aquaculture,2011,315(3-4):269-274.
[23]Itami T,Asano M,Tokushige K,et al.Enhancement of disease resistance of kuruma shrimp,Penaeus japonicus,after oral administration of peptidoglycan derived from Bifidobacterium thermophilum[J].Aquaculture,1998,164(1-4):277-288.
[24]Coteur G,Warnau M,Jangoux M,et al.Reactive oxygen species (ROS)production by amoebocytes of Asterias rubens(Echinodermata)[J].Fish&Shellfish Immunology,2002,12(3):187-200.
[25]Dolmatova L S,Eliseikina M G,Romashina V V.Antioxidant enzymatic activity of coelomocytes of the Far East sea cucumber Eupentacta fraudatrix[J].Journal of Evolutionary Biochemistry and Physiology,2004,40(2):126-135.
[26]Canesi L.Pro-oxidant and antioxidant processes in aquatic invertebrates[J].Annals of the New York Academy of Sciences,2015,1340(1):1-7.
[27]Yang Shiping,Wu Zaohe,Jian Jichang,et al.Effect of marine red yeast Rhodosporidium paludigenum on growth and antioxidant competence of Litopenaeus vannamei[J].Aquaculture,2010,309 (1-4):62-65.
[28]Shen Wenying,Fu Linglin,Li Weifen,et al.Effect of dietary supplementation with Bacillus subtilis on the growth performance,immune response and antioxidant activities of the shrimp(Litopenaeus vannamei)[J].Aquaculture Research,2010,41(11):1691-1698.
[29]Frengova G I,Beshkova D M.Carotenoids from Rhodotorula and Phaffia:yeasts of biotechnological importance[J].Journal of Industrial Microbiology&Biotechnology,2009,36(2):163-180.
[30]Ushakumari U N,Ramanujan R.Isolation of astaxanthin from marine yeast and study of its pharmacological activity[J].International Current Pharmaceutical Journal,2013,2(3):67-69.
[31]王吉桥,樊莹莹,徐振祥,等.饲料中β-胡萝卜素和虾青素添加量对仿刺参幼参生长及抗氧化能力的影响[J].大连海洋大学学报,2012,27(3):215-220.
中图分类号:Q939.9;S968.9
文献标志码:A
DOI:10.16535/j.cnki.dlhyxb.2016.03.007
文章编号:2095-1388(2016)03-0266-06
收稿日期:2015-09-24
基金项目:国家 “863”计划重大项目 (2012AA10A412);农业部北方海水增养殖重点实验室开放课题 (2014-MSENC-KF-06)
作者简介:李璐瑶 (1990—),女,硕士研究生。E-mail:405852333@qq.com
通信作者:马悦欣 (1963—),女,博士,教授。E-mail:mayuexin@dlou.edu.cn
Effects of dietary supplementation of probiotcs on immune response and antioxidant performance in juvenile sea cucumber Apostichopus japonicus
LI Lu-yao1,SUN Pi-hai2,BAO Peng-yun1,ZHANG Cong-yao1,YANG Zhi-ping3,MA Yue-xin1
(1.Key Laboratory of Mariculture&Stock Enhancement in North China’s Sea,Ministry of Agriculture,Dalian Ocean University,Dalian 116023,China;2.Seafood Seedling Breeding Base,Dalian Ocean University,Dalian 116023,China;3.Dalian Huixin Titanium Equipment Development Co. Ltd.,Dalian 116039,China)
Abstract:A feeding trial was conducted to evaluate effects of probiotics on immune response and antioxidant performance in juvenile sea cucumber Apostichopus japonicus.The juvenile sea cucumber with body weight of(0.54± 0.06)g were fed basal diet or diet supplemented with probiotics containing Rhodotorula sp.H26(1×105cells/g diet),Metschnikowia sp.C14(1×105cells/g diet)and Bacillus sp.BC26(1×107cells/g diet)for 4 and 8 weeks. Results showed that there were significantly higher phagocytic activity and respiratory burst in coelomocytes and significantly higher lysozyme and phenoloxidase activities in coelomic fluid(CF)and coelomocytes cells lysate supernatant(CLS)in the sea cucumber fed the diet containing probiotics than those in the control(P<0.05)in 4 weeks. All immune parameters mentioned above were siginificantly enhanced by probiotcs administration compared to the animals in the control(P<0.05)in 8 weeks except for phenoloxidase activity.Superoxide dismutase(SOD)acivity in CF,intestine and body wall,catalase(CAT)acivity and total antioxidant capacity(T-AOC)in all tissues and glutathione(GSH)content in intestine and respiratory tree in sea cucumber fed the diet supplemented with probiotics were shown to be significantly higher than those in the animals fed basal diet(P<0.05)in 4 weeks.SOD activity in intestine,respiratory tree and body wall,CAT activity in all tissues,T-AOC in CLS and intestine and GSH content in CF,intestine and respiratory tree were significantly elevated in the sea cucumber fed the diet supplemented with probiotcs compared to those in the control(P<0.05)in 8 weeks.The findings revealed that diet supplemented with probiotics improved immune response and antioxidant performance in sea cucumber juveniles.
Key words:Apostichopus japonicus;probiotics;immune response;antioxidant performance

