罗非鱼Tilapia隶属于鲈形目 Perciformes、鲡鱼科 Cichidae、罗非鱼属Oreochromis,主要分布于热带、亚热带地区,是具有较高经济价值的暖水性鱼类,由于其繁殖力强、生长快、抗逆性好、肉质鲜嫩、刺少等优点,联合国粮农组织已将罗非鱼作为优良的养殖品种向全世界推广[1-2]。目前,养殖罗非鱼的国家和地区已经超过131个,主要分布于亚洲、美洲和非洲,中国是全球最大的罗非鱼生产国,年养殖产量约为158万t,占世界总产量的三分之一以上,中国罗非鱼养殖区主要集中在广东、海南和广西3省区,产量占全国总产量的79.4%[3]。
无乳链球菌Streptococcus agalactiae又称B簇链球菌(group B streptococci,GBS),是革兰氏阳性球菌,能引起新生儿脑膜炎、败血症、肺炎,以及奶牛乳腺炎,可感染多种水生动物[4]。近年来,随着水产养殖业的发展,无乳链球菌已对多种海、淡水鱼类造成严重危害[5-6]。无乳链球菌最敏感的宿主是罗非鱼,每年均在中国广东、广西、海南、福建等地暴发流行,该病传染力强、致死率高,给水产养殖产业带来巨大的经济损失[7-9]。本研究中,主要从罗非鱼无乳链球菌的病原学、流行病学、病理学、致病机制、检测方法、分子分型、耐药性及疫苗等方面进行系统阐述,旨在为罗非鱼无乳链球菌病防控提供有价值的参考资料。
1 罗非鱼无乳链球菌病的研究概况
1.1 病原特征
无乳链球菌属芽孢杆菌纲Bacilli、乳杆菌目Lactobacillales、链球菌科Streptococcaceae、链球菌属Streptococcus,大多呈球形,排列成链状,菌体直径为0.5~2.0 μm。该菌兼性厌氧,具有荚膜和鞭毛,无芽孢,氧化酶呈阳性,而过氧化氢酶呈阴性[10]。在脑心浸液琼脂固体培养基上生长形成卵圆形且表面光滑的菌落,无乳链球菌多数具有β溶血性,但少部分呈现α溶血或γ溶血性[11],易于在温度为28~37 ℃、pH 为7.4~7.6、盐度为0.85%~3.5%的条件下生长[12]。在GenBank上检索发现,无乳链球菌的基因组平均长度为2.09 MB,编码2000多个蛋白。
1.2 流行病学
对亚洲和拉丁美洲等主要罗非鱼养殖区的链球菌病流行病学调查发现,2001—2009年,82%的分离菌株为无乳链球菌,而海豚链球菌Streptococcus iniae只占18%(www.thefishsite.com/articles/ 812/streptococcosis-in-tilapiaa-more-complex-problem/)。近十年来,无乳链球菌病在巴西[13]、美国[14]、泰国[15]、越南、哥伦比亚、洪都拉斯、哥斯达黎加等多个国家的罗非鱼养殖场暴发流行[16]。在中国,张新艳等[12]首次报道了罗非鱼无乳链球菌病,此后,该病在中国南方的罗非鱼养殖区频繁暴发[10, 17-18]。罗非鱼无乳链球菌病主要流行于夏季,发病高峰期在5—9月份,发病水温为25~35 ℃,尤其当养殖密度较大时,该病极易暴发[10]。起初认为,罗非鱼无乳链球菌主要感染对象为成鱼或鱼种,对体质量小于100 g的鱼不致病,经调查发现,无乳链球菌也能导致100 g以下的仔鱼发病,表明无乳链球菌的感染力在逐渐增强[13,19]。据统计,2008年后,中国罗非鱼链球菌病90%以上是由无乳链球菌引起的[20]。
1.3 临床症状与病理学
无乳链球菌感染罗非鱼的临床症状主要包括离群慢游、间歇性打转、食欲不振、体色发黑、眼球突起、出血、角膜白浊,且鳃盖下缘、胸鳍基部、体表呈现充血或斑块状出血(图1)。解剖病变主要表现为脑膜充血或出血,肝脏呈浅黄色或肿胀出血,胆囊、脾脏和肾脏肿大,部分鱼体肠道发炎、腹腔充满淡黄色液体(图1)。组织病理变化主要表现为:典型的脑膜炎,炎区小胶质细胞增生,脑血管内易形成微血栓;肝脏细胞空泡变性,肝周隙炎性细胞浸润、坏死;肾间质出血,肾小管上皮细胞坏死,管腔内蛋白渗出;心肌炎性水肿,心外膜增厚伴随炎症细胞浸润;脾脏败血性病变,呈多灶性坏死;鳃丝上皮细胞脱落,基部细胞增生,鳃静脉扩张、充血;肠道固有层炎症反应(图2)。无乳链球菌偶尔会与海豚链球菌混合感染罗非鱼,通过比较发现,罗非鱼混合感染的临床症状及病理损伤与单独感染无乳链球菌的症状类似[21]。此外,存在无乳链球菌慢性感染罗非鱼的现象,目前仅发现于成鱼中,发病鱼体表症状不明显,不会致死,在靠近脊椎骨的肌肉内可观察到黄色或暗红色结节,呈现典型的肉芽肿病理学变化[22]。
1.4 感染途径与致病机理
在自然环境中,无乳链球菌可通过饲料、濒死鱼和粪便等进行水平传播,而在实验室条件下,采用肌肉、腹腔注射、浸泡、灌胃、鳃鼻等器官的接种及共栖等途径均可感染罗非鱼[19,23]。目前,尚未见该病原从亲本到子代间垂直传播的报道。无乳链球菌的主要致病机制是躲避吞噬细胞的免疫防御,部分细菌被黏附吞噬后可在巨噬细胞内存活、生长和繁殖,随巨噬细胞在体内游走、释放,扩散感染其他组织,并透过血-脑屏障,进一步损坏脑-神经系统[24]。研究发现,在浸泡条件下,无乳链球菌可在罗非鱼胃肠管腔内定植,并侵入宿主体内发展为全身感染,这可能是罗非鱼感染无乳链球菌的一种重要的致病机制[25]。近些年来,学者通过比较基因组、蛋白质组学和组织病理学等方法来研究强弱毒株的毒力差异,揭示了强毒株在罗非鱼体内的定植和组织损伤规律[26],对无乳链球菌荚膜[27]、转铁蛋白[28]、富含丝氨酸重复蛋白(Srr-1)[29]和neuA基因[30]等功能进行研究,发现其在感染过程中发挥重要作用,但对其他20多个毒力基因缺乏深入的研究。罗非鱼无乳链球菌病的发生与发展不仅与病原体的侵入途径、毒力和数量有关,还决定于宿主自身和养殖环境状况。罗非鱼不同选育品种抗病能力存在明显差异,如无乳链球菌更易于感染吉富罗非鱼[31];具有良好免疫力的罗非鱼更能抵御无乳链球菌的侵染,如通过饲喂植物提取物等措施可提高罗非鱼的天然免疫力,无乳链球菌对机体的侵袭性明显降低[32]。环境因素在无乳链球菌致病过程中起着重要的助推作用,研究显示,在高密度、高温、高亚硝酸盐、高氨氮、低溶解氧及pH值紊乱等[33-34]养殖环境下,无乳链球菌对罗非鱼的侵染力更强。
2 无乳链球菌的检测方法
目前,国内外研究者开发了多种无乳链球菌检测方法,传统细菌生理生化鉴定及免疫学方法操作复杂,检测耗时,达不到快速检测的目的,近20年来,无乳链球菌的检测方法发展迅速,基于分子生物学的检测技术被广泛运用(图3)。
2.1 生理生化检测
金黄色葡萄球菌分泌的β溶血素能够与无乳链球菌产生的“CAMP因子”发生反应,会增强无乳链球菌的溶血性。利用此特性研究者开发出CAMP检测方法,但检测时间长,操作烦琐[35]。法国拜奥默里克斯公司研发出基于酯酶活性且高灵敏的无乳链球菌检测技术,特异性较好,但比较耗时[36]。

注:A为不规则游动(箭头);B为眼球突出、充血(箭头);C为角膜白浊(星号);D为脑膜出血(箭头);E为肝脏呈浅黄色(箭头),肾脏肿大(白星号),肠壁发炎(星号);F为肝脏充血、出血(星号),脾脏肿大(箭头),胆囊肿大(白星号)
Note:A, Erratic swimming (arrow);B, Exophthalmia and congestion (arrow);C, Corneal opacity (asterisk);D, Meningeal hemorrhage (arrow);E, Light yellow liver (arrow), enlarged kidney (white asterisk), and inflammation in the gut wall (asterisk); F, Congestion and hemorrhage of the liver (asterisk), enlarged spleen (arrow) and gallbladder (white asterisk)
图1 无乳链球菌感染罗非鱼的临床症状和剖检病变
Fig.1 Clinical symptoms and necropsy lesions of tilapia infected with Streptococcus agalactiae
2.2 免疫学检测
胶乳法建立在抗原抗体结合的基础上,利用该方法检测无乳链球菌具有较高的敏感性和特异性,该方法操作简便、易于判定反应结果,但检验中需对病原菌进行分离纯化,才能做进一步检测,由于试剂的价格比较昂贵,不易于商业推广[37]。
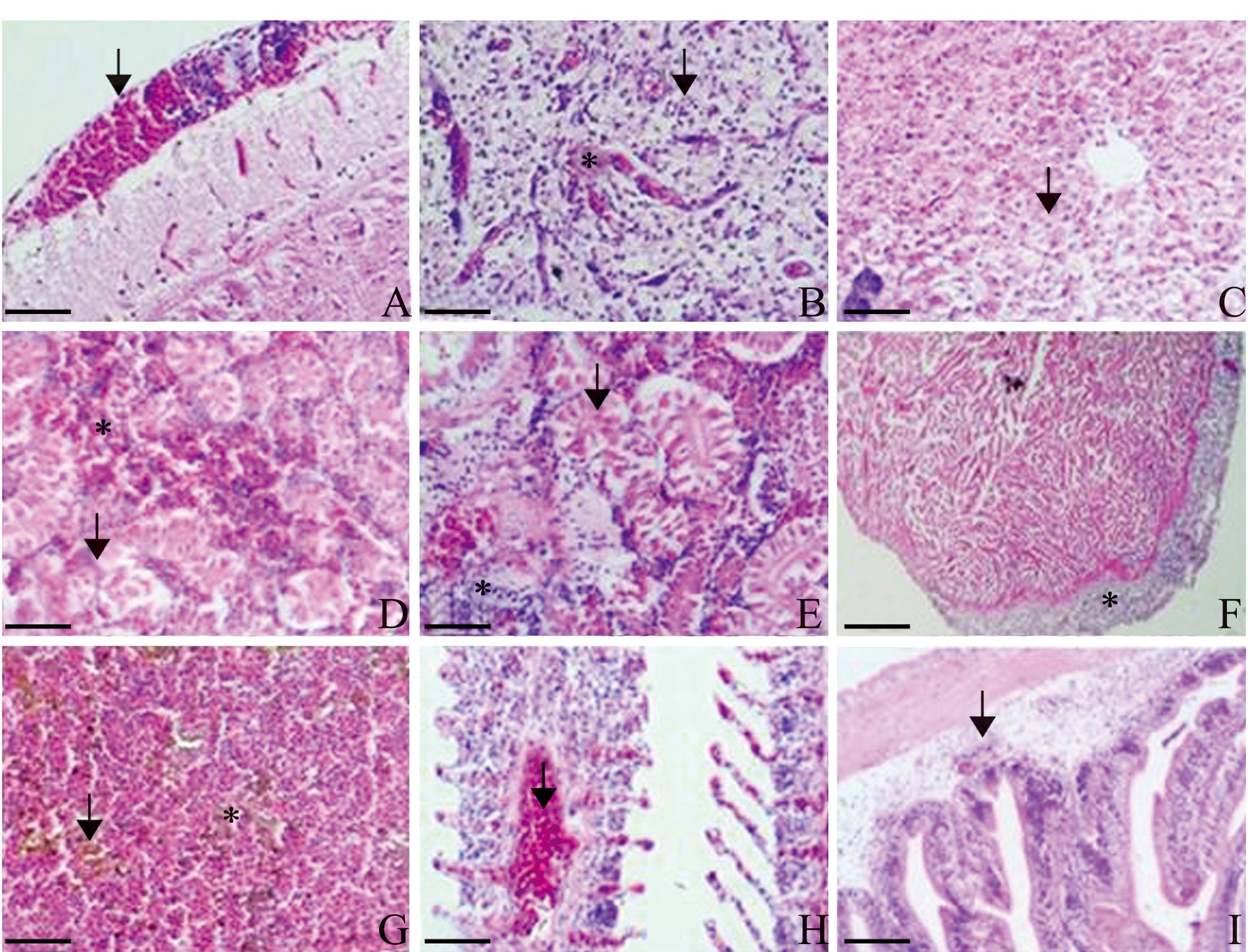
注:A为脑膜增厚伴随炎症细胞浸润(箭头);B为脑小胶质细胞增生(箭头)和微血栓形成(星号);C为肝细胞空泡变性和坏死(箭头);D为肾间质出血(星号),肾小管降解(箭头);E为肾小管上皮细胞坏死,蛋白渗出(箭头),炎症细胞浸润(星号);F为心外膜增厚伴随炎症细胞浸润(星号);G为脾脏多灶性坏死(箭头和星号);H为鳃丝基部细胞增生,静脉扩张、充血(箭头);I为肠道固有层水肿、充血、炎症细胞浸润(箭头)。标尺:A~H=50 μm,I=100 μm
Note:A,Thickening and congestion of the meninges with inflammatory cells infiltration (arrow); B, Hyperplasia of microglial cells (arrow) and microvenous thrombosis (asterisk); C, Vacuolar degeneration and necrosis of hepatocytes (arrow); D, Severe hemorrhage in renal interstitium (asterisk) and dissolution of tubules (arrow); E, Necrotic tubular epithelial cells, fibrin precipitation (arrow) and leucocytes infiltration (asterisk); F, Epicardium thickening with amounts of inflammatory cells infiltration (asterisk); G, Severe multifocal necrotizing splenitis (arrow and asterisk); H, Hyperplasia in the gill lamella base with central venous sinus enlarging and congesting (arrow); I, Severe edema, congestion and inflammatory cells infiltration in lamina propria (arrow).Bar:A-H=50 μm,I=100 μm
图2 无乳链球菌感染罗非鱼的组织病理学[23]
Fig.2 Histopathology of tilapia infected with Streptococcus agalactiae[23]
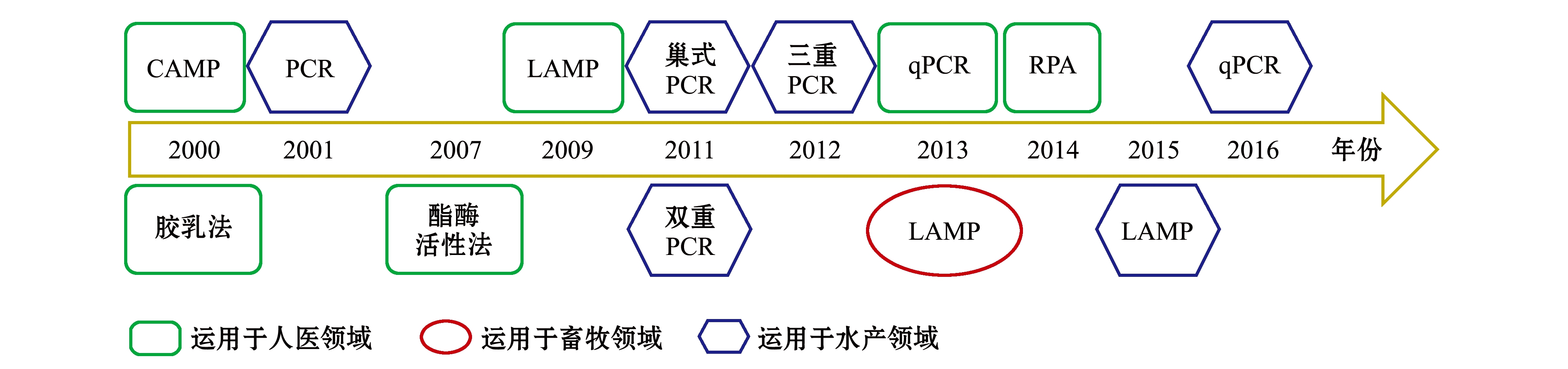
图3 无乳链球菌检测技术发展示意图
Fig.3 Schematic diagram of the development process of Streptococcus agalactiae detection technology
2.3 PCR检测
Berridge等[38]以无乳链球菌的16S-23S rDNA间区序列为靶标,建立了快捷、灵敏的PCR检测方法,之后,学者们又构建了无乳链球菌的巢式PCR[39]、双重PCR[40]和三重PCR[41]检测技术,进一步提高了检测的灵敏度和特异性。无乳链球菌的荧光定量PCR(qPCR)检测方法由于具有敏感高、特异性好、无污染等优点,已被广泛应用于医学领域的产前筛查[42],目前,qPCR也被用于罗非鱼无乳链球菌的检测[43]。
2.4 环介导等温扩增技术 (Loop-mediated isothermal amplification,LAMP)
LAMP是在恒温体系下通过链置换型DNA聚合酶作用进行核酸扩增的一种新型检测技术,其对引物要求较高,需在靶标基因的6个保守区上设计出4条特异引物。LAMP操作简便、特异性强,仅需肉眼即可鉴定检测结果。无乳链球菌的LAMP检测方法已被广泛应用于医学[44]、畜牧[45]和水产[46]等领域。
2.5 重组酶聚合酶扩增技术 (Recombinase polymerase amplification,RPA)
RPA是最近几年新开发的一种检测方法,仅在常温条件下,20 min以内即可完成对靶基因的扩增。该技术仪器设备简单、操作简便且检测时间短,适合于食品安全、医学和农业等领域的快速检测。学界已有运用RPA技术检测细菌、病毒和寄生虫等病原微生物的报道[47]。目前,利用RPA检测无乳链球菌仅见于医学领域,而在畜牧和水产领域尚未见报道[48]。
3 罗非鱼无乳链球菌的分子分型
目前,现有的无乳链球菌分型方法有多种,如分子血清型、多位点可变数目串联重复序列分析(Multiple-locus variable number tandem repeat analysis,MLVA)、多位点序列分型 (Multilocus sequence typing,MLST)、脉冲场凝胶电泳 (Pulsed field gel electrophoresis,PFGE)和毒力基因型分型等。无乳链球菌的分型有助于了解各菌株间的同源及相关性,获得来源不同宿主的菌株分子遗传多样性信息,理清菌株的流行和传播规律,解析流行菌株是否存在交叉感染性,同时也可为筛选疫苗候选菌株提供参考。
3.1 分子血清型
分子血清型已经成为研究无乳链球菌流行病学的重要工具。按照荚膜多糖基因簇的变化特征,把无乳链球菌分为Ⅰa、Ⅰb和Ⅱ~Ⅸ共10种血清型[49]。目前,罗非鱼无乳链球菌Ⅰa型在全球广泛流行[9,16],Ⅰb型主要在巴西养殖区暴发流行,而该地区流行的Ⅲ型菌株则为突发性致病菌,Ⅲ型菌株存在人-鱼共患的风险[13]。流行于中国养殖区的罗非鱼无乳链球菌主要血清型为Ⅰa型,Ⅰb型菌株仅在少数地区流行,罗非鱼无乳链球菌的Ⅰa和Ⅰb型菌株间含有一些与免疫相关的差异蛋白[50-51],Ⅲ型菌株则流行时间短且暴发的范围比较局限[9,52]。Zhang等[53]报道,分离自广东养殖区的80株无乳链球菌中有73株为Ⅲ型,7株为Ⅸ型,且Ⅸ型为在罗非鱼中新发现的血清型,这与其他研究者的检测结果存在较大差异,笔者认为,需要用不同的方法和更多样品去检测无乳链球菌Ⅲ型和Ⅸ型在中国罗非鱼养殖区的流行状况。另有研究证实,无乳链球菌的所有血清型中,Ⅲ 型菌株最易感染新生儿[54-55],Ⅰa型菌株对兔子和奶牛则较敏感[56]。Kayansamruaj等[1]研究发现,从养殖环境中分离的Ⅲ型无乳链球菌并不感染罗非鱼,而人源无乳链球菌Ⅰa、Ⅲ和Ⅴ型菌株均可导致罗非鱼发病[57]。
3.2 MLVA和MLST技术
研究无乳链球菌在分子水平上的差异时最常用的方法是MLVA和MLST技术。Zhang等[58]对流行于2007—2012年不同地区罗非鱼源无乳链球菌进行分子分型后发现,所有菌株均为同一MLVA型,表明在不同地区和年份,分离的无乳链球菌起源相同,而牛源无乳链球菌具有多种MLVA分型。MLST分型主要用于展示菌群生物学变异及进化状况,利用该方法可把罗非鱼无乳链球菌分为9种MLST型,即ST-7、ST-260、ST-261、ST-283、ST-491、ST-500、ST-891、NST-1和NST-2[9,59-60]。在中国,罗非鱼无乳链球菌的主要优势菌群为ST-7型,仅少部分菌株为ST-261和 ST-891型。另发现,血清型为Ⅰa型的罗非鱼无乳链球菌的MLST分型基本是ST-7,而血清型为Ⅰb的菌株则为ST-261型[8]。罗非鱼无乳链球菌还存在Ⅰa-ST-891型菌株[60],起初认为,在中国也存在罗非鱼无乳链球菌NST-1和NST-2两种新型,但最终确认是ST-7的克隆复合物[61]。由此可见,罗非鱼无乳链球菌菌株间存在一定的遗传变异性,但尚处于较低的变异水平[9,60]。Delannoy等[16]发现,泰国的罗非鱼无乳链球菌存在Ⅰa型-ST-500和Ⅲ型-ST-283,而在越南分离到Ⅲ型-ST-491菌株,这表明,不同国家间的罗非鱼无乳链球菌在MLST分型结果上存在一定的差异。学者对不同来源的无乳链球菌进行MLST分型发现,强致病性的人源无乳链球菌极有可能来源自牛源菌株,并揭示牛源和人源菌株间存在较高的交叉感染性[56,62]。也有学者认为,牛源和鱼源无乳链球菌为不同的STs 型,它们之间不可能存在交叉感染性[1],这与Lusiastuti等[63]的研究结论相同。
3.3 PFGE分型技术
PFGE在细菌的分子分型中具有较高的辨识度[64],但其试验数据重复性不高,为了增加病原菌分子流行病学研究结果的准确性,通常结合MLST的方法进行综合分析。研究表明,相同ST型和血清型的无乳链球菌在PFGE脉冲场带型中表现出高度聚集现象,表明PFGE技术可辅助菌株的MLST分型和血清型鉴定[60]。大量的PFGE分型结果显示,鱼源无乳链球菌流行株的基因型多样性显著,能区分出流行菌株的地理分布和发生改变的时间节点,这将有助于筛选出有效的疫苗候选株,为罗非鱼无乳链球菌疫苗的研制提供便利[20,65-66]。
3.4 毒力基因分型
毒力基因分型是检测菌株携带或缺失某些毒力基因,根据细菌毒力基因谱特征而进行菌株分型。人源和牛源无乳链球菌的ST型和分子血清型的多样性较高,但鱼源无乳链球菌则相对单一,利用菌株毒力基因谱的差异将有助于鱼源无乳链球菌的分型。毒力基因分型结果显示,罗非鱼无乳链球菌均携带PI-1+PI-2b基因,缺失PI-2a基因,截至2011年,PI-2b型无乳链球菌是中国罗非鱼养殖区的主要流行株,而近几年的流行株则转变为PI-1+PI-2b型,中国与国外的罗非鱼无乳链球菌毒力基因型不完全一致[1,67]。张德锋等[9]通过对毒力基因分型发现,中国的罗非鱼无乳链球菌Ⅰa和Ⅰb型菌株的bac、bca 和cylE基因存在差异,Ⅲ 型菌株携带bca基因,但缺失bac基因,而国外的Ⅰb-ST-260/261型菌株均缺失bac和bca基因[16]。同其他陆生动物源无乳链球菌相比,罗非鱼无乳链球菌普遍缺失lmb 和scpB基因[68]。
4 罗非鱼无乳链球菌的耐药性
罗非鱼暴发链球菌病时,由于缺乏高效疫苗,抗菌药物仍然是养殖基层防控该病的首选,长期用药和滥用药会加快无乳链球菌对某些抗生素产生不同程度的耐药性。总体来看,罗非鱼无乳链球菌对链霉素、新霉素、庆大霉素、磺胺甲基异恶唑、复方新诺明等药物基本耐药。近些年来,菌株对氨苄西林、环丙沙星等抗菌药的耐药率普遍增加(表1)。由于养殖环境和用药习惯的差异,无乳链球菌的耐药性呈现出一定的区域差异。Liu等[69]对广东地区近6年罗非鱼无乳链球菌菌株进行药物敏感分析发现,所有菌株存在着多重耐药性,有多达23种耐药谱型,对氨基糖苷类、磺胺类、吡哌酸和诺氟沙星等药的耐药率超过90%,且菌株耐药谱种类随时间不断扩大更新。另有研究显示,在福建和海南养殖区分离的无乳链球菌对氯霉素和四环素具有较高耐药率[7,70]。而在巴西,罗非鱼无乳链球菌对氨苄西林、喹诺酮类、磺胺类和四环素均耐药,且呈现出多重耐药性和耐药谱单一的特点[13]。了解无乳链球菌的耐药规律,对于指导合理使用抗菌药,防止多重耐药菌株流行和扩散,保障水产品质量安全具有重要意义。
5 罗非鱼无乳链球菌的疫苗研究
疫苗不仅是防控养殖鱼类发病的有效途径,同时还可保障水产品质量安全和水环境安全。近20年来,罗非鱼无乳链球菌疫苗的研究进展迅速,疫苗的种类主要包括灭活全菌苗、重组疫苗、减毒疫苗和DNA疫苗[71]。从1995年的首个无乳链球菌灭活苗被成功研制[72],到将无乳链球菌、佐剂和基础饲料等混合制备成的饲料型灭活疫苗[73],已有多种灭活苗被陆续应用到罗非鱼养殖过程中。无乳链球菌灭活苗成本低廉,免疫保护效果好,适合大规模商业化应用,但存在流行菌株血清型改变导致保护率降低的现象。随着全基因组测序、蛋白质组等新技术的兴起,已筛选出细胞壁表面锚蛋白[74]、甘油醛-磷酸脱氢酶蛋白[75]等多种无乳链球菌免疫原性蛋白用于制备重组疫苗,重组疫苗可针对不同血清型的无乳链球菌,但免疫效果普遍不及灭活苗高,此外,该疫苗用量大、成本高,在商品化生产过程中受限。减毒疫苗能有效诱导免疫应答,通常在低剂量下就达到终生免疫,目前,仅少量的无乳链球菌减毒疫苗被开发,如利用抗生素筛选的多价苗[76]和利用连续传代致弱的减毒疫苗[77],虽然这些疫苗对罗非鱼具有稳定的免疫保护力,尤其对仔鱼更明显,但罗非鱼是一种重要的水产品,必须确保减毒疫苗对人类及环境的安全性,目前,开发商业化的无乳链球菌减毒疫苗阻力较大。罗非鱼无乳链球菌DNA疫苗正逐渐受到研究者关注,多个研究团队报道,基于sip基因设计的DNA疫苗具有较好免疫保护效果[78-79],Huang等[80]发现,该口服DNA疫苗的免疫保护率高于灭活全菌苗,表明以sip蛋白为靶标的DNA疫苗具有良好的应用前景。最近,Wang等[81]研制了一种新型无乳链球菌菌蜕疫苗,该疫苗不仅能够显著诱导罗非鱼的体液和细胞免疫反应,其对鱼体的免疫保护力也高于灭活疫苗,该研究为罗非鱼无乳链球菌疫苗开发提供了新的方向。
表1 2006—2016年不同地区分离的罗非鱼无乳链球菌耐药率比较
Tab.1 Comparison of drug resistant rate of Streptococcus agalactiae isolated from tilapia in different regions from 2006 to 2016
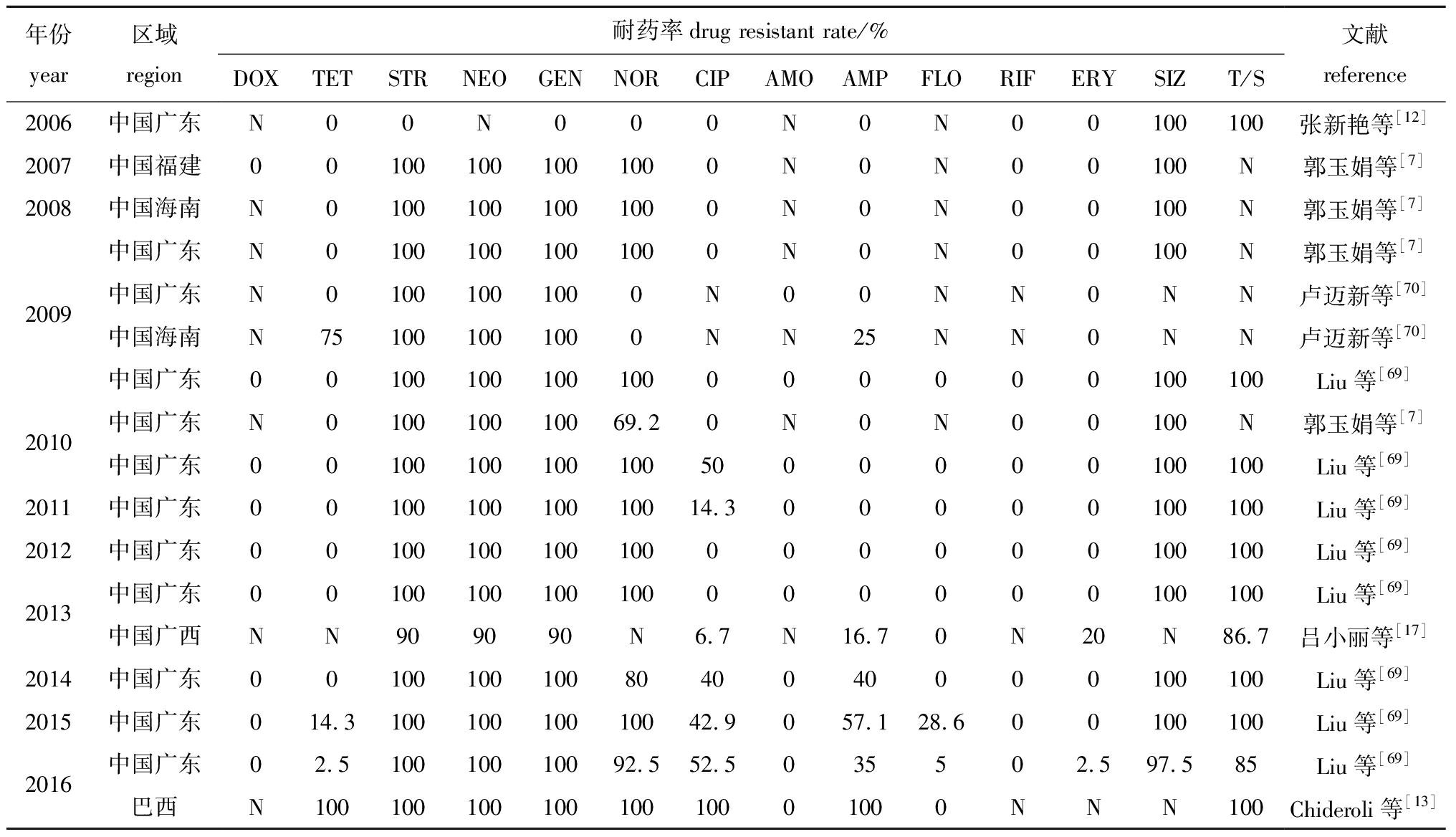
年份year区域region耐药率drug resistant rate/% DOXTETSTRNEOGENNORCIPAMOAMPFLORIFERYSIZT/S文献reference2006中国广东N00N000N0N00100100张新艳等[12]2007中国福建001001001001000N0N00100N郭玉娟等[7]2008中国海南N01001001001000N0N00100N郭玉娟等[7]中国广东N01001001001000N0N00100N郭玉娟等[7]2009中国广东N01001001000N00NN0NN卢迈新等[70]中国海南N751001001000NN25NN0NN卢迈新等[70]中国广东00100100100100000000100100Liu等[69]2010中国广东N010010010069.20N0N00100N郭玉娟等[7]中国广东001001001001005000000100100Liu等[69]2011中国广东0010010010010014.300000100100Liu等[69]2012中国广东00100100100100000000100100Liu等[69]2013中国广东00100100100100000000100100Liu等[69]中国广西NN909090N6.7N16.70N20N86.7吕小丽等[17]2014中国广东001001001008040040000100100Liu等[69]2015中国广东014.310010010010042.9057.128.600100100Liu等[69]2016中国广东02.510010010092.552.5035502.597.585Liu等[69]巴西N10010010010010010001000NNN100Chideroli等[13]
注:N为无数据;DOX为强力霉素;TET为四环素;STR为链霉素;NEO为新霉素;GEN为庆大霉素;NOR为诺氟沙星;CIP为环丙沙星;AMO为阿莫西林;AMP为氨苄西林;FLO为氟苯尼考;RIF为利福平;ERY为红霉素;SIZ为磺胺甲基异恶唑;T/S为复方新诺明
Note:N, No data; DOX, doxitard; TET, tetracycline; STR, streptomycin; NEO, neomycin; GEN, gentamicin; NOR, norfloxacin; CIP, ciprofloxacin; AMO, amoxicillin; AMP, ampicillin; FLO, florfenicol; RIF, rifampin; ERY, erythromycin; SIZ, sulfafurazole; T/S, trimethoprim/sulfamethoxazole
疫苗的免疫效果与接种途径密切相关,目前,针对罗非鱼的疫苗接种方式主要有腹腔注射、浸泡、喷雾和口服等[82]。浸泡免疫成本低,操作便利,可同时对大量个体进行免疫接种,且不会由于人为操作因素导致鱼体死亡和免疫抑制[77,83]。喷雾也是一种比较简便的免疫方式,目前仅有1篇文献报道该接种途径可对罗非鱼无乳链球菌感染提供一定的免疫保护[84],但仍需更多试验数据为后续的开发提供支撑。口服途径通常是罗非鱼无乳链球菌减毒疫苗和DNA疫苗采用免疫接种方式,与浸泡疫苗类似,口服疫苗操作方便,但该疫苗抗原在通过消化道时会被一些酶类破坏,导致疫苗的免疫保护力降低,限制了该疫苗的商业化应用[77-78,85]。与上述几种免疫途径相比,腹腔注射疫苗无须穿越鱼体的黏膜屏障即可快速诱导机体启动获得性免疫反应,还可根据保护效果来优化抗原剂量。综上所述,腹腔注射可对罗非鱼提供更高的免疫保护力,也是目前被普遍采用的免疫接种方式[77,83],但该接种途径最大的缺点是对鱼个体注射时操作烦琐,耗时耗力,还会对鱼产生应激反应。近年来,随着专业化免疫接种服务团队和半自动化注射装置的出现,注射途径也逐渐便利化,这将有助于高效注射疫苗在罗非鱼养殖中的商品化推广[71]。
6 存在问题及展望
罗非鱼无乳链球菌病危害较大,虽然国内外学者对其进行了大量研究,但仍有以下几方面的问题急需解决。
(1)传统药物使用受限需开发绿色新药物。药物仍然是防控罗非鱼无乳链球菌病的主要手段,但长期使用化学药物和抗生素不仅对环境造成污染,还会引起细菌的耐药性,虽然无乳链球菌对磺胺类药物表现出严重耐药性,在防控罗非鱼病害过程中仍然会被大量使用,这也是造成罗非鱼“磺胺超标事件”的一个重要原因;中草药被认为是“绿色环保抗菌剂”,可用于防控无乳链球菌病,但需对中草药的成分和药理作用进行科学评价。
(2)疫苗开发和商业化应用出现瓶颈需开辟新的研制途径。环保和无抗养殖将决定罗非鱼养殖的发展方向,接种疫苗是未来预防和控制罗非鱼无乳链球菌病的最佳选择,但罗非鱼无乳链球菌疫苗从研究到临床应用仍有较长的路要走,腹腔注射接种方式操作难度大,急需加强新型疫苗的研发,特别是口服和浸泡疫苗,此外,罗非鱼无乳链球菌病的疫苗需要安全高效、生产成本低且使用方便,这样才有利于商业化推广。目前,通常采用腹腔注射致病菌及测定相对保护率的手段来评价罗非鱼无乳链球菌疫苗的保护效果,由于直接把病原菌强行注射到鱼体内,从而忽略了对细菌从黏膜器官侵入过程的疫苗免疫保护能力评价,今后的研究中应把建立自然感染模型作为重点,进一步阐明疫苗在细菌定植宿主黏膜、穿越各系统屏障,以及扩散至靶器官并造成相应病理损伤过程中的免疫保护机制。
(3)罗非鱼无乳链球菌基础研究薄弱需加强致病机制等方面的研究力度。从源头上解决罗非鱼无乳链球菌造成的影响及危害,必须加强对该菌致病机制的深入研究,尤其是毒力基因和传播途径两方面,这将有助于找到有效防控该病的策略;无乳链球菌分子分型的方法较多,但目前还没有更好的技术对罗非鱼无乳链球菌菌株多样性进行细分,这也是开发高效疫苗受阻的原因之一,因此急需开发出更加精细的分子分型方法;无乳链球菌检测方法较多,加强对亲鱼、鱼苗和养殖环境的病原检测是净化该病的有效途径,分子生物学技术的飞速发展以及大量罗非鱼无乳链球菌基因组序列的公布,促进了对无乳链球菌基因组特征、流行病学、病原学、检测方法和防控措施等方面的研究,为有效防控甚至净化该病提供了理论依据;在罗非鱼养殖过程中盲目追求经济效益最大化,养殖密度不断提高,生态环境受到严重破坏,条件致病菌和有害细菌大量滋生,导致罗非鱼体质下降,抗应激能力差,因此,应降低罗非鱼养殖密度,调控优良的生态环境,从生态角度寻求新方法也是防控无乳链球菌病的有效途径。
[1] Kayansamruaj P,Pirarat N,Katagiri T,et al.Molecular characterization and virulence gene profiling of pathogenic Streptococcus agalactiae populations from tilapia (Oreochromis sp.) farms in Thailand[J].Journal of Veterinary Diagnostic Investigation,2014,26(4):488-495.
[2] 李荣妮,唐瑞波,朱莉飞,等.饲料中添加蚯蚓粉和蚯蚓粪对罗非鱼生长及血清抗氧化指标的影响[J].大连海洋大学学报,2018,33(2):233-238.
[3] 农业农村部渔业渔政管理局.2018中国渔业统计年鉴[M].北京:中国农业出版社,2018.
[4] Wang Rui,Li Liping,Huang Yin,et al.Pathogenicity of human ST23 Streptococcus agalactiae to fish and genomic comparison of pathogenic and non-pathogenic isolates[J].Frontiers in Microbiology,2017,8:1933.
[5] 刘礼辉,张德锋,李宁求,等.鲮鱼源无乳链球菌的鉴定、血清型分析及药敏试验[J].南方农业学报,2015,46(11):2053-2058.
[6] Barony G M,Tavares G C,Pereira F L,et al.Large-scale genomic analyses reveal the population structure and evolutionary trends of Streptococcus agalactiae strains in Brazilian fish farms[J].Scientific Reports,2017,7(1):13538.
[7] 郭玉娟,张德锋,樊海平,等.中国南方地区罗非鱼无乳链球菌的分子流行病学研究[J].水产学报,2012,36(3):399-406.
[8] 张德锋,可小丽,刘志刚,等.中国七种水生动物源无乳链球菌的分子特征及其对斑马鱼的致病性[J].水产学报,2017,41(11):1788-1797.
[9] 张德锋,袁伟,可小丽,等.中国罗非鱼主养区无乳链球菌的分子流行特征及其传播方式[J].中国水产科学,2017,24(3):606-614.
[10] Ye Xing,Li Jiong,Lu Maixin,et al.Identification and molecular typing of Streptococcus agalactiae isolated from pond-cultured tilapia in China[J].Fisheries Science,2011,77(4):623-632.
[11] Delannoy C M J,Zadoks R N,Lainson F A,et al.Draft genome sequence of a nonhemolytic fish-pathogenic Streptococcus agalactiae strain[J].Journal of Bacteriology,2012,194(22):6341-6342.
[12] 张新艳,樊海平,钟全福,等.罗非鱼无乳链球菌的分离、鉴定及致病性研究[J].水产学报,2008,32(5):772-779.
[13] Chideroli R T,Amoroso N,Mainardi R M,et al.Emergence of a new multidrug-resistant and highly virulent serotype of Streptococcus agalactiae in fish farms from Brazil[J].Aquaculture,2017,479:45-51.
[14] Evans J J,Klesius P H,Pasnik D J,et al.Human Streptococcus agalactiae isolate in Nile tilapia (Oreochromis niloticus)[J].Emerging Infectious Diseases,2009,15(5):774-776.
[15] Suanyuk N,Kong Fanrong,Ko D,et al.Occurrence of rare genotypes of Streptococcus agalactiae in cultured red tilapia Oreochromis sp. and Nile tilapia O.niloticus in Thailand—relationship to human isolates?[J].Aquaculture,2008,284(1-4):35-40.
[16] Delannoy C M J,Crumlish M,Fontaine M C,et al.Human Streptococcus agalactiae strains in aquatic mammals and fish[J].BMC Microbiology,2013,13:41.
[17] 吕小丽,黎姗梅,彭民毅,等.广西罗非鱼主养区致病性链球菌及其耐药性调查[J].广西畜牧兽医,2017,33(3):143-148.
[18] 邝伟键,黄良宗,刘明杰,等.罗非鱼源无乳链球菌的分离鉴定及药敏试验[J].畜牧与兽医,2017,49(9):62-66.
[19] 祝璟琳,杨弘.鱼源无乳链球菌致病机理研究进展[J].广东海洋大学学报,2013,33(6):92-96.
[20] Chen Ming,Li Liping,Wang Rui,et al.PCR detection and PFGE genotype analyses of streptococcal clinical isolates from tilapia in China[J].Veterinary Microbiology,2012,159(3-4):526-530.
[21] Chen Chunyao,Chao C B,Bowser P R.Comparative histopathology of Streptococcus iniae and Streptococcus agalactiae-infected tilapia[J].Bulletin of the European Association of Fish Pathologists,2007,27:1-9.
[22] Li Y W,Liu L,Huang P R,et al.Chronic streptococcosis in Nile tilapia,Oreochromis niloticus (L.),caused by Streptococcus agalactiae[J].Journal of Fish Diseases,2014,37(8):757-763.
[23] Soto E,Zayas M,Tobar J,et al.Laboratory-controlled challenges of Nile tilapia (Oreochromis niloticus) with Streptococcus agalactiae:comparisons between immersion,oral,intracoelomic and intramuscular routes of infection[J].Journal of Comparative Pathology,2016,155(4):339-345.
[24] Wang Zhaofei,Guo Changming,Xu Yannan,et al.Two novel functions of hyaluronidase from Streptococcus agalactiae are enhanced intracellular survival and inhibition of proinflammatory cytokine expression[J].Infection and Immunity,2014,82(6):2615-2625.
[25] Iregui C A,Comas J,Vásquez G M,et al.Experimental early pathogenesis of Streptococcus agalactiae infection in red tilapia Oreochromis spp.[J].Journal of Fish Diseases,2016,39(2):205-215.
[26] Su Youlu,Feng Juan,Liu Chan,et al.Dynamic bacterial colonization and microscopic lesions in multiple organs of tilapia infected with low and high pathogenic Streptococcus agalactiae strains[J].Aquaculture,2017,471:190-203.
[27] Barato P,Martins E R,Vasquez G M,et al.Capsule impairs efficient adherence of Streptococcus agalactiae to intestinal epithelium in tilapias Oreochromis sp.[J].Microbial Pathogenesis,2016,100:30-36.
[28] Poochai W,Choowongkomon K,Srisapoome P,et al.Characterization and expression analysis of the transferrin gene in nile tilapia (Oreochromis niloticus) and its upregulation in response to Streptococcus agalactiae infection[J].Fish Physiology and Biochemistry,2014,40(5):1473-1485.
[29] Liu Guangjin,Zhang Wei,Liu Yongjie,et al.Identification of a virulence-related surface protein XF in piscine Streptococcus agalactiae by pre-absorbed immunoproteomics[J].BMC Veterinary Research,2014,10:259.
[30] Wang E L,Wang K Y,Chen D F,et al.Molecular cloning and bioinformatic analysis of the Streptococcus agalactiae neuA gene isolated from tilapia[J].Genetics and Molecular Research,2015,14(2):6003-6017.
[31] 祝璟琳,邹芝英,李大宇,等.四个罗非鱼选育品种抗链球菌病能力差异研究[J].水生生物学报,2017,41(6):1232-1241.
[32] Kareem Z H,Abdelhadi Y M,Christianus A,et al.Effects of some dietary crude plant extracts on the growth and gonadal maturity of Nile tilapia (Oreochromis niloticus) and their resistance to Streptococcus agalactiae infection[J].Fish Physiology and Biochemistry,2016,42(2):757-769.
[33] Amal M N A,Saad M Z,Zahrah A S,et al.Water quality influences the presence of Streptococcus agalactiae in cage cultured red hybrid tilapia,Oreochromis niloticus×Oreochromis mossambicus[J].Aquaculture Research,2015,46(2):313-323.
[34] Delphino M K V C,Leal C A G,Gardner I A,et al.Seasonal dynamics of bacterial pathogens of Nile tilapia farmed in a Brazilian reservoir[J].Aquaculture,2019,498:100-108.
[35] 杨梦,洪秀华,魏民,等.金黄色葡萄球菌与枯草杆菌间的类似CAMP现象[J].上海第二医科大学学报,2000,20(2):189-190.
[36] 胡瑞萍,刘磊,边艳青,等.无乳链球菌检测及其奶牛乳腺炎防治的研究进展[J].中国兽药杂志,2008,42(6):54-57.
[37] 苏桂华,兰宇,张军民.胶乳法快速检测无乳链球菌的评价[J].上海医学检验杂志,2000,15(5):310.
[38] Berridge B R,Bercovier H,Frelier P F.Streptococcus agalactiae and Streptococcus difficile 16S-23S Intergenic rDNA:genetic homogeneity and species-specific PCR[J].Veterinary Microbiology,2001,78(2):165-173.
[39] Jiménez A,Tibatá V,Junca H,et al.Evaluating a nested-PCR assay for detecting Streptococcus agalactiae in red tilapia (Oreochromis sp.) tissue[J].Aquaculture,2001,321(3-4):203-206.
[40] 王均,汪开毓,肖丹,等.罗非鱼源无乳链球菌双重PCR快速检测方法的建立[J].中国兽医科学,2011,41(5):496-502.
[41] 黄锦炉,汪开毓,肖丹,等.无乳链球菌(Streptococcus agalactiae)三重PCR快速检测方法的建立与应用[J].海洋与湖沼,2012,43(2):254-261.
[42] Park J S,Cho D H,Yang J H,et al.Usefulness of a rapid real-time PCR assay in prenatal screening for group B streptococcus colonization[J].Annals of Laboratory Medicine,2013,33(1):39-44.
[43] Su Y L,Feng J,Li Y W,et al.Development of a quantitative PCR assay for monitoring Streptococcus agalactiae colonization and tissue tropism in experimentally infected tilapia[J].Journal of Fish Diseases,2016,39(2):229-238.
[44] 王永,赵新,景海春,等.LAMP检测无乳链球菌方法的建立及应用[J].华北农学报,2009,24(5):234-238.
[45] Kimura K,Yanagisawa H,Wachino J I,et al.Rapid and reliable loop-mediated isothermal amplification method for detecting Streptococcus agalactiae[J].Japanese Journal of Infectious Diseases,2013,66(6):546-548.
[46] 郑磊,樊海平,吴斌,等.罗非鱼无乳链球菌LAMP快速检测方法的建立[J].福州大学学报:自然科学版,2015,43(4):572-576.
[47] Lobato I M,O’Sullivan C K.Recombinase polymerase amplification:basics,applications and recent advances[J].TrAC Trends in Analytical Chemistry,2018,98:19-35.
[48] Daher R K,Stewart G,Boissinot M,et al.Isothermal recombinase polymerase amplification assay applied to the detection of group B streptococci in vaginal/anal samples[J].Clinical Chemistry,2014,60(4):660-666.
[49] Imperi M,Pataracchia M,Alfarone G,et al.A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae[J].Journal of Microbiological Methods,2010,80(2):212-214.
[50] 石云良,李莉萍,王瑞,等.罗非鱼源Ⅰa和Ⅰb血清型无乳链球菌可溶性蛋白差异分析[J].大连海洋大学学报,2015,30(2):125-131.
[51] 李莉萍,王瑞,黄婷,等.2007-2012年中国罗非鱼无乳链球菌流行菌株血清型分析[J].大连海洋大学学报,2014,29(5):469-475.
[52] Li Liping,Wang Rui,Liang Wanwen,et al.Rare serotype occurrence and PFGE genotypic diversity of Streptococcus agalactiae isolated from tilapia in China[J].Veterinary Microbiology,2013,167(3-4):719-724.
[53] Zhang Ze,Lan Jiangfeng,Li Yuhui,et al.The pathogenic and antimicrobial characteristics of an emerging Streptococcus agalactiae serotype IX in tilapia[J].Microbial Pathogenesis,2018,122:39-45.
[54] Wang Ping,Ma Zhuoya,Tong Jingjing,et al.Serotype distribution,antimicrobial resistance,and molecular characterization of invasive group B Streptococcus isolates recovered from Chinese neonates[J].International Journal of Infectious Diseases,2015,37:115-118.
[55] 周燕珍,王利民,方寅飞.无乳链球菌致新生儿血流感染的血清型与耐药性研究[J].中国卫生检验杂志,2017,27(8):1190-1193.
[56] 耿毅,张雨薇,汪开毓,等.牛源、兔源及人源无乳链球菌分子分型特征分析[J].中国预防兽医学报,2017,39(11):880-885.
[57] Chen Ming,Wang Rui,Luo Fuguang,et al.Streptococcus agalactiae isolates of serotypes Ia,Ⅲ and V from human and cow are able to infect tilapia[J].Veterinary Microbiology,2015,180(1-2):129-135.
[58] Zhang Defeng,Li Aihua,Guo Yujuan,et al.Molecular characterization of Streptococcus agalactiae in diseased farmed tilapia in China[J].Aquaculture,2013,412-413:64-69.
[59] Lusiastuti A M,Textor M,Seeger H,et al.The occurrence of Streptococcus agalactiae sequence type 261 from fish disease outbreaks of tilapia Oreochromis niloticus in Indonesia[J].Aquaculture Research,2014,45(7):1260-1263.
[60] 张雨薇,耿毅,余泽辉,等.鱼源无乳链球菌的血清型及分子分型研究[J].水生生物学报,2017,41(4):800-806.
[61] 王蓓,黎源,陈贺,等.我国华南地区罗非鱼源无乳链球菌分子流行病学研究[J].水产科学,2014,33(12):741-749.
[62] Bisharat N,Crook D W,Leigh J,et al.Hyperinvasive neonatal group B Streptococcus has arisen from a bovine ancestor[J].Journal of Clinical Microbiology,2004,42(5):2161-2167.
[63] Lusiastuti A M,Seeger H,Indrawati A,et al.The comparison of Streptococcus agalactiae isolated from fish and bovine using multilocus sequence typing[J].HAYATI Journal of Biosciences,2013,20(4):157-162.
[64] Pinto T C A,Costa N S,Souza A R V,et al.Distribution of serotypes and evaluation of antimicrobial susceptibility among human and bovine Streptococcus agalactiae strains isolated in Brazil between 1980 and 2006[J].The Brazilian Journal of Infectious Diseases,2013,17(2):131-136.
[65] Chen Ming,Wang Rui,Li Liping,et al.Screening vaccine candidate strains against Streptococcus agalactiae of tilapia based on PFGE genotype[J].Vaccine,2012,30(42):6088-6092.
[66] Li L,Shi Y,Wang R,et al.Proteomic analysis of tilapia Oreochromis niloticus Streptococcus agalactiae strains with different genotypes and serotypes[J].Journal of Fish Biology,2015,86(2):615-636.
[67] 袁伟,张德锋,可小丽,等.中国罗非鱼主养区无乳链球菌流行菌株的菌毛岛屿及其血清型分型[J].中国预防兽医学报,2018,40(6):490-494.
[68] Kayansamruaj P,Pirarat N,Kondo H,et al.Genomic comparison between pathogenic Streptococcus agalactiae isolated from Nile tilapia in Thailand and fish-derived ST7 strains[J].Infection,Genetics and Evolution,2015,36:307-314.
[69] Liu Chan,Feng Juan,Zhang Defeng,et al.Clustering analysis of antibiograms and antibiogram types of Streptococcus agalactiae strains from tilapia in China[J].Microbial Drug Resistance,2018,24(9):1431-1439.
[70] 卢迈新,黎炯,叶星,等.广东与海南养殖罗非鱼无乳链球菌的分离、鉴定与特性分析[J].微生物学通报,2010,37(5):766-774.
[71] Liu Guangjin,Zhu Jielian,Chen Kangming,et al.Development of Streptococcus agalactiae vaccines for tilapia[J].Diseases of Aquatic Organisms,2016,122(2):163-170.
[72] Eldar A,Shapiro O,Bejerano Y,et al.Vaccination with whole-cell vaccine and bacterial protein extract protects tilapia against Streptococcus difficile meningoencephalitis[J].Vaccine,1995,13(9):867-870.
[73] Ismail M S,Syafiq M R,Siti-Zahrah A,et al.The effect of feed-based vaccination on tilapia farm endemic for streptococcosis[J].Fish & Shellfish Immunology,2017,60:21-24.
[74] Liu H,Zhang S,Shen Z,et al.Development of a vaccine against Streptococcus agalactiae in fish based on truncated cell wall surface anchor proteins[J].Veterinary Record,2016,179(14):359.
[75] Zhang Ze,Yu Angen,Lan Jiangfeng,et al.GapA,a potential vaccine candidate antigen against Streptococcus agalactiae,in Nile tilapia (Oreochromis niloticus)[J].Fish & Shellfish Immunology,2017,63:255-260.
[76] Pridgeon J W,Klesius P H.Development of live attenuated Streptococcus agalactiae as potential vaccines by selecting for resistance to sparfloxacin[J].Vaccine,2013,31(24):2705-2712.
[77] Li L P,Wang R,Liang W W,et al.Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro[J].Fish & Shellfish Immunology,2015,45(2):955-963.
[78] Zhu Ling,Yang Qian,Huang Lingyuan,et al.Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia[J].Developmental & Comparative Immunology,2017,77:77-87.
[79] Ma Yanping,Ke Hao,Liang Zhiling,et al.Protective efficacy of cationic-PLGA microspheres loaded with DNA vaccine encoding the sip gene of Streptococcus agalactiae in tilapia[J].Fish & Shellfish Immunology,2017,66:345-353.
[80] Huang L Y,Wang K Y,Xiao D,et al.Safety and immunogenicity of an oral DNA vaccine encoding sip of Streptococcus agalactiae from Nile tilapia Oreochromis niloticus delivered by live attenuated Salmonella typhimurium[J].Fish & Shellfish Immunology,2014,38(1):34-41.
[81] Wang Qishuo,Wang Xuepeng,Wang Xuemei,et al.Generation of a novel Streptococcus agalactiae ghost vaccine and examination of its immunogenicity against virulent challenge in tilapia[J].Fish & Shellfish Immunology,2018,81:49-56.
[82] Munang’andu H M,Paul J,Evensen Ø.An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus)[J].Vaccines,2016,4(4):48.
[83] Evans J J,Klesius P H,Shoemaker C A.Ef cacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreochromis niloticus) by intraperitoneal and bath immersion administration[J].Vaccine,2004,22(27-28):3769-3773.
cacy of Streptococcus agalactiae (group B) vaccine in tilapia (Oreochromis niloticus) by intraperitoneal and bath immersion administration[J].Vaccine,2004,22(27-28):3769-3773.
[84] Noraini O,Sabri M Y,Siti-Zahrah A.Efficacy of spray administration of formalin-killed Streptococcus agalactiae in hybrid red tilapia[J].Journal of Aquatic Animal Health,2013,25(2):142-148.
[85] Zhang Lei,Zeng Zhanzhuang,Hu Chaohua,et al.Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines[J].Biomaterials,2016,77:307-319.